
Ԫ���ȣ�Sr��λ�����ڱ���5���ڵ�IIA�塣̼���ȣ�SrCO3�����㷺���ڲ�ɫ���ӻ����������߹ܡ�������ʯ����Ҫ�ɷ�ΪSrSO4�������������ʣ�Ϊԭ����ȡ�ߴ�̼���ȵġ����ս�ȡ�������ֹ�������ʾ�����¡�
��1��д����Ԫ����ͬ���3����Ԫ�ص�ԭ�ӽṹʾ��ͼ�� ��
��2�������������±����У���0.5mol SrSO4��ֻ��S����ԭ��ת����4mol���ӡ�д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3����ȡ�ߴ�̼���ȹ����У������Ҵ�����HOCH2CH2NH2����ΪCO2���ռ����Ҵ���ˮ��Һ�������ԣ���NH3����ˮԭ�����ƣ���ԭ���� �������ӷ���ʽ��ʾ����
��4���������й���2֮���г��ӵȶಽ������������������� ��
��5��������ʪ������һ����ȡ̼���ȵķ�����������ʯ��ĩ��̼������Һ��ϣ�70oC�¼���1��2h��һϵ�в������õ�̼���ȡ���Ҫ��ӦΪ�� SrSO4��s��+CO
SrSO4��s��+CO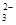 ��aq�� SrCO3��s��+SO
��aq�� SrCO3��s��+SO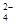 ��aq��
��aq��
[��֪��70oCʱ��Ksp��SrSO4��=3��3��10��7��Ksq��SrCO3��=1��1��10��10]
������Ӧ��ƽ�ⳣ��K= ��
��6��ͨ��������ʪ�����õ���̼���ȴ��Ƚϵͣ����ܵ�ԭ���� ��дһ�֣���
��14�֣���1��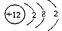 ��2�֣�
��2�֣�
��2��SrSO4+4C SrS+4CO����3�֣�
SrS+4CO����3�֣�
��3��HOCH2CH2NH2+H2O HOCH2CH2NH3++OH����3�֣�
HOCH2CH2NH3++OH����3�֣�
��4��ϴ�ӡ���ɣ�2�֣�
��5��3��103��2�֣�
��6������������ʯ��ԭ�еIJ��������ʣ���SrSO4(s)+CO32��(aq) SrCO3(s)+SO42��(aq)�ǿ��淴Ӧ��SrCO3�бض�����SrSO4����2�֣�
SrCO3(s)+SO42��(aq)�ǿ��淴Ӧ��SrCO3�бض�����SrSO4����2�֣�
���������������1����3���ڵ�IIA��Ԫ��Ϊþ���˵����Ϊ+12��������Ӳ�ṹΪ282����2�����������֪����Ԫ�صĻ��ϼ���+6��Ϊ��2�����ݵ��ӡ�ԭ���غ��֪���÷�ӦʽΪSrSO4+4C SrS+4CO������3��NH3+H2O
SrS+4CO������3��NH3+H2O NH3?H2O
NH3?H2O NH4++OH�����������Կ�֪��HOCH2CH2NH2+H2O
NH4++OH�����������Կ�֪��HOCH2CH2NH2+H2O HOCH2CH2NH3++OH������4������֮����Ҫϴ�ӳ�������ȥ�������������Ŀ����Ȼ���ٸ����ȥˮ�֣���5���������⣬K=
HOCH2CH2NH3++OH������4������֮����Ҫϴ�ӳ�������ȥ�������������Ŀ����Ȼ���ٸ����ȥˮ�֣���5���������⣬K=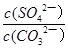 =
=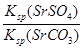 =
=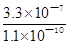 =3��103����6��SrSO4(s)+CO32��(aq)
=3��103����6��SrSO4(s)+CO32��(aq) SrCO3(s)+SO42��(aq)�ǿ��淴Ӧ��SrCO3�бض�����SrSO4�Ȳ��������ʡ�
SrCO3(s)+SO42��(aq)�ǿ��淴Ӧ��SrCO3�бض�����SrSO4�Ȳ��������ʡ�
���㣺�������ʽṹ��Ԫ�����ڱ���������ԭ��Ӧ�����ӷ���ʽ�������ķ�����ᴿ���ܶȻ��ͻ�ѧƽ�ⳣ���ļ��㡢������ת�������֪ʶ��


 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ���¯����������ȡ��������������������Դ����Դ�����ʡ���¯���ͻ�����߲�ҵ���ж��Լ������ȷ��滹�����ʴ��ںܴ��࣬�д���һ����ߣ�Ŭ�������ǿ��������
��1����¯������ԭ��������̿��ʯ��ʯ���������ۼ����õ��� ��Ŀ���dz�ȥ����ʯ�е���ʯ��������ܶȱ��� ����������ˮ�� ����ϲ������²������γ�¯��������ˮ���롣
��2����̿�ڸ�¯���������ž������ص����ã����в����ڽ�̿���õ��� ��
| A����Ϊȼ�ϣ�Ϊ�����еĻ�ѧ��Ӧ��Ӧ��ɾȥ���ṩ���� |
| B����Ϊ��ԭ���������̼��Ӧ������ԭ��������һ����̼ |
| C���Ը�¯�е�������֧�ź���ɢ������ |
| D����Ϊ�ۼ�����ȥ����ʯ�е����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ѺϽ��Ǻ��캽�չ�ҵ����Ҫ���ϡ�����������Ҫ�ɷ�Ϊ������������ѧʽΪFeTiO3���Ʊ�Ti�Ȳ�Ʒ��һ�ֹ�������ʾ��ͼ���£�
��֪���� TiO2����ˮ�⣬ֻ�ܴ�����ǿ������Һ�С� �ڳ����£����ܵ�����ܽ����pH��ϵͼ��
��25 ��ʱTiO(OH)2�ܶȻ�Ksp=1��10-29
�ش��������⣺
��1��д�����������ʱ����Ӧ�����ӷ���ʽ ��
��2������������ľ������������ ��
��3������TiO2����Һ���м���Na2CO3��ĩ�������� �� TiO2��ˮ������ӷ���ʽΪ ������ҺpH= ʱ��TiO(OH)2�ѳ�����ȫ��
��4��������м��Fe3+ת��ΪFe2+��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�ϣ���������Ϊԭ���Ʊ��������ѵ�ij������������ͼ��ʾ����������Ҫ�ɷ�
Ϊ��������( FeTiO3)������һ������Ԫ���ڷ绯�����л�ת��Ϊ+3�ۡ�
���������գ�
��1��������У������۽�Fe3��ת��ΪFe2���ķ�Ӧ�����ӷ���ʽΪ____��
��2��������У�ʵ�ֻ����ķ������������ʵ�____������ĸ��ţ���
a���۷е���� b�ܽ��Բ��� c�����ԡ���ԭ�Բ���
��3������ڡ��ۡ����У�������еIJ�����____����������ƣ���
��4�����������������еķ�Һ�����̿���Ҫ�ɷ�MnO2����Ӧ���������̣���Ӧ�����ӷ���ʽΪ
��5��������ͼװ�ã�ʯī������������������������CaF2��CaO������ʣ��ɻ�ý����ƣ�������Ϊ��ԭ�����ɻ�ԭ���������Ƹ������ѡ�
�������������ķ�ӦΪ________��
�����Ʊ�������ǰ��CaO���������䣬��ԭ����____��
��6��_Tҵ����4.0���������Ƶ�1. 6�ֵĶ������ѣ�������������Ԫ�ص�����������____��
������������������û����ʧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ⷨ���Ѱ۲����ķ�Һ[���д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4]����������Ͳ�Ѫ�������������������������£�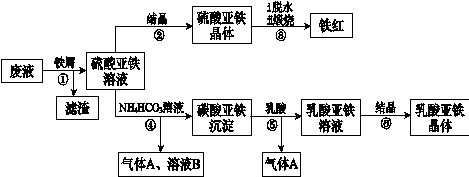
��֪��TiOSO4������ˮ����ˮ�п��Ե���ΪTiO2+��SO42������ش�
��1��������з�������������Һ�������IJ��������õIJ��������� ��
����ڵõ�������������IJ���Ϊ����Ũ���� ��
��1������ܵ����ӷ���ʽ�� ��
��1������ޱ������һ������նȣ�ԭ��������������ˮ�Լ� ��
��1�����������ڿ�������������������������÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ ��
��1����ƽ���ƶ���ԭ�����Ͳ�����м������ܵõ�����������ԭ�� ��
��1��Ϊ�ⶨ����������þ�����FeSO4��7H2O������������ȡ������Ʒa g������ϡ�������100��00 mL��Һ��ȡ��20��00 mL��Һ����KMnO4��Һ�ζ���������KMnO4����Ӧ����������0��1000 mol?L-1 KMnO4��Һ20��00 mL�����þ�����FeSO4��7H2O����������Ϊ����a��ʾ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
TiO2�׳��Ѱۣ���;�㷺�������㷺�����������л����Ϊ�¹�ҵ����ҵ������TiO2�Ʊ������ѵ��������¡�
��֪��I��
Ti(s)��O2(g)=TiO2(s) ��H=��946 kJ��mol��1
2C(s)��O2(g)=2CO(g) ��H=��221 kJ��mol��1
Ti(s)��2Cl2(g)=TiCl4(g) ��H=��815 kJ��mol��1
II��ij�¶��¸����ʵķе����£�
| ���� | TiCl4 | FeCl3 | SiCl4 | AlCl3 |
| �е�/�� | 136 | 310 | 56.5 | 180 |
 TiCl4(g)+2CO (g) ���Լ����䷴Ӧ�ġ�H= kJ?mol��1����Ӧ��ƽ�ⳣ������ʽK= ������ͼ������TiCl4�ﵽƽ���ٷֺ������¶ȵı仯����ͼ��
TiCl4(g)+2CO (g) ���Լ����䷴Ӧ�ġ�H= kJ?mol��1����Ӧ��ƽ�ⳣ������ʽK= ������ͼ������TiCl4�ﵽƽ���ٷֺ������¶ȵı仯����ͼ��
 TiCl4��l��+O2��g����H=+151kJ?mol��1���÷�Ӧ�ڸ��������µ����Է�����������̼��Ӧ��˳�����У��Խ�������ԭ��
TiCl4��l��+O2��g����H=+151kJ?mol��1���÷�Ӧ�ڸ��������µ����Է�����������̼��Ӧ��˳�����У��Խ�������ԭ�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�ǹ�ҵ������̼��﮵IJ��ֹ������̣����������ͼ����֪��Ϣ�ش����⡣
��
�ۼ������ʲ�ͬ�¶��µ��ܽ�ȡ�
��1��������1�з����Al2O3�IJ�����������ͼ��ʾ�����ű�ʾ������Լ��������ʾ���õ������ʡ�д��ͼ�Т١��ڡ��۱�ʾ�ĸ����ʣ�����II�з�Ӧ�����ӷ���ʽ�� ��
��2����֪����2����Ҫ�ɷ���Mg��OH��2��CaCO3��д����������2��Ӧ�����ӷ���ʽ��
��
��3������Һ2�м��뱥��Na2CO����Һ�����˺��á���ˮϴ�ӡ���ԭ���� ��
��4����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ������¡�
�ٽ��ֲ�ƷLi2CO3�����������ý�۵�����Һ��LiOH��Һ������Һ������������ѡ���Ĥ�������ö��Ե缫��⡣�����ĵ缫��Ӧʽ�� ��
�ڵ������ƷLiOH��Һ�м������NH4HCO����Һ����Li2CO3��Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ԭ���ܹ�ҵ������Դ��ҵ�е���Ҫ���ʡ���ҵ�ϳ��æ¡���﮿�(��Ҫ�ɷ���LiAlSi2O6�����������ơ�þ����)�Ʊ�����ﮣ��������������£�
��֪Li2CO3����ˮ����ش��������⣺
��1��д��LiAlSi2O6�����ᷴӦ�Ļ�ѧ����ʽ_______________________��
��2������B����Ҫ�ɷ���_____________(д��ѧʽ)��
��3������Ũ��Li2SO4��Һʱ����Ҫʹ�õĹ�������������������_________��_________��
��4��������������������Ũ��Li2SO4��Һ��Ŀ����____________________��
��5�����������﮵��ʱ������FeF3���������Ļ������ʣ�FeF3����FeCl3��40%HF��Һ��Ӧ�Ʊ������Ʊ���������Ҫѡ�����ƾ��ķ���ϩ���ϵ��������з�Ӧ������������ͨ�IJ����������մ���������ԭ����_________________________________(�û�ѧ��Ӧ����ʽ��ʾ)��
��6������﮿����ڴ�����������ԭ���ǣ���2Li��H2��2LiH����LiH��H2O��LiOH��H2��������֪LiH���ܶ�Ϊ0.82g��cm-3���ý��������224L H2����״����ǡ����ȫ��Ӧ�������ɵ�LiH������뱻���յ����������֮��Ϊ1: ______(��ȷ������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�йؽӴ������������������ȷ���ǣ���������
| A����Ǻ������������Ϊ����ԭ�� |
| B������ʯ���飬��Ϊ����߿�ʯ�������� |
| C����Ӧ��ͨ������Ŀ�����Ϊ�����FeS2��SO2��ת���� |
| D�������������������ᾭŨ������Ա�ɷ������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com