
| 22��24ag |
| 24 |
| 27ag��48 |
| 54 |
| 22��24ag |
| 24 |
| 24ag��2 |
| 24 |
| 22��24ag |
| 24 |
| 48n |
| 6 |


 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ú�����������������ú���ۺ�����Ч��[ |
| B����������ú��ʯ�ͣ���߲��������㹤ҵ�����Ŀ��ٷ�չ |
| C������̫���ܡ�ˮ�ܡ����ܡ���ȼ��������Դ������ʹ��ú��ʯ�͵Ȼ�ʯȼ�� |
| D��ʵ����Դ�ġ�3R�����ùۣ�����������Դ���ģ�Reduce����������Դ���ظ�ʹ�ã�Reuse������Դ��ѭ��������Recycle�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ϩ�Ͷ��� |
| B������Ͷ�ϩ |
| C������Ͷ�ϩ |
| D�������2-����ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѡ�Լ� | �б� | |
| ����һ | п | ȡ������Ʒ�ֱ���п��Ӧ���������������ϡ���ᣬû�е����Ȼ�����Һ�� |
| ������ | ||
| ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
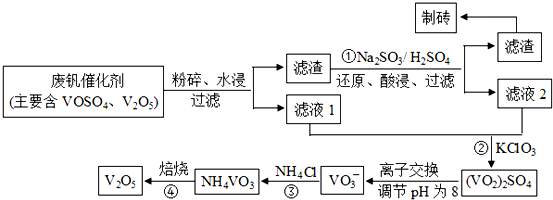
| ���� | V2O5 | VOSO4 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
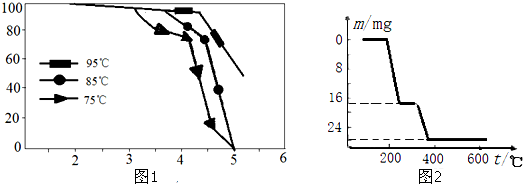
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ͭ��Һ | B��Ũ���� |
| C��ϡ���� | D����������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1��16 | B��16��1 |
| C��7��17 | D��7��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���� | B���� | C���ڢ� | D���ڢۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com