
��һ����ܱ�������ʢ��1 mol PCl5�����ȵ�200�棬������Ӧ��PCl5(g)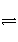
 PCl3(g)��Cl2(g)����Ӧ�ﵽƽ��ʱ��PCl5�ڻ�������е��������Ϊm%��������ͬ���¶Ⱥ���ͬ�������У���ʼʱ����2 mol PCl5����Ӧ�ﵽƽ��ʱ��PCl5�ڻ�������е��������Ϊn%����m��n�Ĺ�ϵ��ȷ����(����)
PCl3(g)��Cl2(g)����Ӧ�ﵽƽ��ʱ��PCl5�ڻ�������е��������Ϊm%��������ͬ���¶Ⱥ���ͬ�������У���ʼʱ����2 mol PCl5����Ӧ�ﵽƽ��ʱ��PCl5�ڻ�������е��������Ϊn%����m��n�Ĺ�ϵ��ȷ����(����)
A��m>n B��m<n C��m��n D�����Ƚ�
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ö��Ե缫���һ����������ͭ��Һ��ʵ��װ��
��ͼ1��ʾ���������е�ʵ��������ͼ2��ʾ���������ʾ��������ת�Ƶ��ӵ����ʵ������������
ʾ�������в����������������������������˵������ȷ����
A���������У�b�缫�������к�ɫ����������Ȼ��������ݲ���
B���ӿ�ʼ��Q��ʱ�ռ����Ļ�������ƽ����Է�������Ϊ17
C������OP �α�ʾH2��O2������� ������仯������PQ�α�ʾO2������仯
������仯������PQ�α�ʾO2������仯
D��a�缫�Ϸ�����Ӧ�ķ���ʽΪ��2H+ + 2e- = H2����4OH-–4 e-=2H2O+ O2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
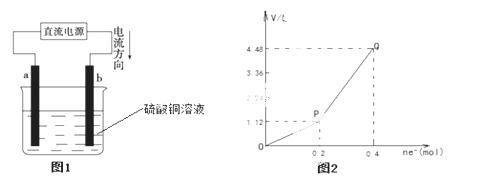
��ʯī�缫���CuCl2��Һ������ͼ�������з�����ȷ����
A��a����ֱ����Դ�ĸ���
B��ͨ��ʹCuCl2��������
C�������Ϸ����ķ�Ӧ��Cu2++2e-=Cu
D��ͨ��һ��ʱ��������������۲쵽����ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
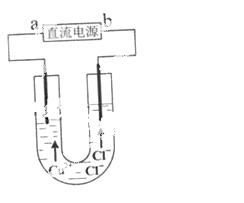
��ͼ��ģ���ȼҵ�����м�������Ƿ�й©��װ��,�����й�˵���������(����)
A.��ƿ���������ְ���
B.��ƿ���������ֺ���ɫ
C.��ƿ�з����ķ�Ӧ���������°����л�ԭ��
D.�ձ��е�NaOH��Һ��Ϊ�������к�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪H2(g)��I2(g)
 2HI(g)����H<0������ͬ�ݻ��Ķ����ܱ��������ң����м���H2��I2��0.1 mol�����м���HI 0.2 mol����ͬ�¶��·ֱ�ﵽƽ��Ũ�ȡ���ʹ����HI��ƽ��Ũ�ȴ�������HI��ƽ��Ũ�ȣ�Ӧ��ȡ�Ĵ�ʩ��(����)
2HI(g)����H<0������ͬ�ݻ��Ķ����ܱ��������ң����м���H2��I2��0.1 mol�����м���HI 0.2 mol����ͬ�¶��·ֱ�ﵽƽ��Ũ�ȡ���ʹ����HI��ƽ��Ũ�ȴ�������HI��ƽ��Ũ�ȣ�Ӧ��ȡ�Ĵ�ʩ��(����)
A���ס��������ͬ�¶�
B�����м���0.1 mol He���Ҳ���
C�������¶ȣ��Ҳ���
D��������0.1 mol H2��������0.1 mol I2������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й���������[��֪N2(g)��3H2(g)
 2NH3(g)����H����92.4 kJ��mol��1]��
2NH3(g)����H����92.4 kJ��mol��1]��
| ���� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1 mol N2�� 3 mol H2 | 2 mol NH3 | 4 mol NH3 |
| NH3��Ũ�� (mol��L��1) | c1 | c2 | c3 |
| ��Ӧ�������仯 | �ų�a kJ | ����b kJ | ����c kJ |
| ��ϵѹǿ(Pa) | p1 | p2 | p3 |
| ��Ӧ��ת���� | ��1 | ��2 | ��3 |
����˵����ȷ����(����)
A��2c1>c3����������������B��a��b��92.4
C��2p2<p3 D����1����3>1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�й�����ʵ�������˵����ȷ����
A��ʵ���ҿ���pH��ֽ����Ũ�����pHֵ
B������ɫ��Ӧʵ��ʱ��ֱ��ȡ�ò�˿պȡ�����ھƾ������������գ��۲�����ɫ
C���ü�ʽ�ζ�����ȡ20.00mL���������Һ
D�������Ȼ�̼��ȡ��ˮ��I2�Ĺ����У�����Һ©����ת������ʹ����Һ���ֽӴ����������ʹ©���ڵ�����ų�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E������ ���о�����ͬһ�ַǽ���Ԫ�أ������ܷ�����ͼ��ʾ��ת����ϵ��
���о�����ͬһ�ַǽ���Ԫ�أ������ܷ�����ͼ��ʾ��ת����ϵ��
��Ԫ��(��R��ʾ)�ĵ�������NaOH��Һ��Ӧ������(Na2RO3)��������
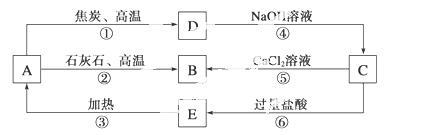
��ش��������⣺
��1�� д�������ʵĻ�ѧʽ��A__________��B__________��C__________��D__________��E__________��
д�������ʵĻ�ѧʽ��A__________��B__________��C__________��D__________��E__________��
��2��д����Ӧ�ٵĻ�ѧ����ʽ��__________________________________________��
�÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ________��
��3��д����Ӧ�ܵ����ӷ���ʽ��_________________________________________��
��4��д����Ӧ�ݵ����ӷ���ʽ��__________________________________________��
��5��H2CO3������ǿ��E�ģ��������ӷ���ʽ����֤����
_____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±�����������ij�����½���þ�����ֱ��������н���������Ӧʱ���ڽ�����������������Ĥ��ʵ���¼��a��b��Ϊ���¶��йصij�����
| ��Ӧʱ��t/h | 1 | 4 | 9 | 16 | 25 |
| MgO���y/nm | 0.05a | 0.20a | 0.45a | 0.80a | 1.25a |
| NiO���y��/nm | b | 2b | 3b | 4b | 5b |
(1)�����ڸ����µ�������ʴ���ʿ����ý�������Ĥ��������������ʾ����������________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
(2)��������Ĥ��Ĥ��y��ʱ��t�����ֵĹ�ϵ�ǣ�MgO����Ĥ��Ĥ��y����________�ͣ�NiO����Ĥ��Ĥ��y��������________�͡�(�ֱ�ߡ��������ߡ���˫���ߡ�������������)
(3)Mg��Ni��Ƚϣ�����________���и��õ���������ʴ�ԣ���������
________________________________________________________________________
______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com