
���� ��������Ҫ�ɷ�ΪAl������������Fe��Mg���ʣ���ѡ��ŨNaOH�ܽ⣬�õ�ƫ��������Һ����ͨ�����˳�ȥFe��Mg�����ʣ���Һ�м���NH4HCO3��Һ�ٽ�AlO2-ˮ������Al��OH��3���������˺����ܽ���ϡ�����У��õ���������Һ������K2SO4��Һ������Ũ������ȴ�ᾧ�õ�����������
��1���������Խ�������ǿ�ᡢǿ�Ӧ����Mg��Feֻ���ܽ����ᣬ�ݴ�ѡ���Լ���
��2����NaOH��Һ�ܽ�Al����ƫ�����Ƽ��������ݴ�д����Ӧ��ѧ����ʽ��
��3����Һ�м���NH4HCO3��Һ�ٽ�AlO2-ˮ������Al��OH��3������Al3+ˮ��ʹ������Һ�����ԣ�
��4��Al��OH��3�����ܽ���NaOH��Һ����ķ�ӦΪAl��OH��3+OH-?AlO2-+2H2O�����ˮ�����ӻ������������ĵ���ƽ�ⳣ������˷�Ӧ��ƽ�ⳣ����
��� �⣺��������Ҫ�ɷ�ΪAl������������Fe��Mg���ʣ���ѡ��ŨNaOH�ܽ⣬�õ�ƫ��������Һ����ͨ�����˳�ȥFe��Mg�����ʣ���Һ�м���NH4HCO3��Һ�ٽ�AlO2-ˮ������Al��OH��3���������˺����ܽ���ϡ�����У��õ���������Һ������K2SO4��Һ������Ũ������ȴ�ᾧ�õ�����������
��1�����������ܽ���ǿ���ǿ������Һ��������þֻ���ܽ���ǿ������Һ�е����ʲ��죬��ѡ��NaOH��Һ�ܽ������ޣ��ɳ�ȥ���е�����þ�����ʣ�
�ʴ�Ϊ��d��
��2����������������Һ��Ӧ����ƫ�����ƺ�������������Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O�T2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����
��3����Һ�м���NH4HCO3��Һ�������NH4+��HCO3-���ܴٽ�AlO2-ˮ�⣬��ӦʽΪNH4++AlO2-+2H2O=Al��OH��3��+NH3•H2O������Al��OH��3��������Al3++3H2O?Al��OH��3+3H+��������ˮ��Һ�����ԣ�
�ʴ�Ϊ��Al��OH��3��Al3+ˮ�⣬ʹ��Һ��H+Ũ������
��4��Al��OH��3?AlO2-+H++H2O��H2O?H++OH-�ڣ���-�ڿɵ�Al��OH��3+OH-?AlO2-+2H2O����Al��OH��3����NaOH��Һ��Ӧ��ƽ�ⳣ��=K��Kw=$\frac{2.0��1{0}^{-13}}{1.0��1{0}^{-14}}$=20��
�ʴ�Ϊ��20��
���� ���������Ʊ�����Ϊ���壬�������ʵ��Ʊ�����ơ��Լ������ᴿʵ��������漰��Ӧԭ����̽�����ܽ�ƽ����ˮ����ƽ�ⳣ����Ӧ�á�����ˮ��ȣ�������֪ʶ�ۺ�Ӧ�õĿ��飬��Ŀ�Ѷ��еȣ��״���Ϊ��4��ƽ�ⳣ���ļ��㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 22.4��������һ�����а����ӵ������������� | |
| B�� | 1Ħ������������32�� | |
| C�� | 1Ħ������1Ħ������̼�е�ԭ�Ӹ������ | |
| D�� | H2SO4��Ħ��������98�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����µ���Դ | B�� | �ϳ��µ����� | ||
| C�� | �о������˶����� | D�� | ���λ�����Ⱦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ������� | H2CO3 | NH3•H2O |
| ����ƽ�ⳣ�� | Ka1=4.30��10-7 Ka2=5.61��10-11 | Kb=1.77��10-5 |
�鿴�𰸺ͽ���>>
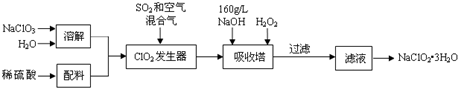
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
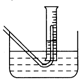 Na2O2��ˮ�ķ�Ӧʵ����Na2O2+2H2O�T2NaOH+H2O2����Ӧ����ʹ����H2O2���ȷֽ⣺2H2O2�T2H2O+O2����Ϊ�˲ⶨij�������ƹ���Ĵ��ȣ�������ʵ�飨�������ʲ��μӷ�Ӧ��
Na2O2��ˮ�ķ�Ӧʵ����Na2O2+2H2O�T2NaOH+H2O2����Ӧ����ʹ����H2O2���ȷֽ⣺2H2O2�T2H2O+O2����Ϊ�˲ⶨij�������ƹ���Ĵ��ȣ�������ʵ�飨�������ʲ��μӷ�Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com