
����Ŀ��һ�ֲⶨˮ���������ӵ�Ũ�ȵ�ʵ�鲽�����£�
������ƿ�м��봦�����ˮ��25.00mL�����뼸��NH4Fe(SO4)2��Һ��
������V1mL c1mol��L-1 AgNO3��Һ�������������ҡ�ȡ�
����c2mol��L-1KSCN����Һ���еζ������յ�ʱ���ı���ҺV2mL ��
����֪��Ksp(AgBr)=7.7��10-13,Ag++SCN-= AgSCN(��ɫ)����Ksp(AgSCN)=1��10-12������˵������ȷ����
A. �ζ��յ�ʱ����Һ��Ϊ��ɫ
B. �õζ������ڼ��������½���
C. AgBr(s)+SCN��![]() AgSCN(s)+Br-(aq����ƽ�ⳣ��K=0.77
AgSCN(s)+Br-(aq����ƽ�ⳣ��K=0.77
D. ��ˮ����������Ũ��Ϊ��c(Br-��=(c1V1-c2V2)/25.00mol/L

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ŀǰ���緶Χ�ڵ���ԴΣ�����״���Ϊһ�ֽϺõĿ�������Դ�����й㷺��Ӧ��ǰ����
(1)��֪�ڳ��³�ѹ�·�Ӧ���Ȼ�ѧ����ʽ��
��CO(g)��2H2(g)CH3OH(g) ��H1�� -90 kJ��mol��1
��CO(g)��H2O(g)CO2(g)��H2(g) ��H2�� -41 kJ��mol��1
д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ��_______________________��
(2)���ݻ�ΪVL�������г���amol CO��2amol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ʱ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��

��p1 ________ p2(����������������������������)��
�����¶ȡ�������������£�������amol CO��2amol H2���ﵽ��ƽ��ʱ��CO��ת����________(����������������С������������)��ƽ�ⳣ��_____(����������������С������������)��
(3)��֪��T��ʱ��CO(g)��H2O(g)CO2(g)��H2(g)��ƽ�ⳣ��K��0.32���ڸ��¶��£���֪cʼ(CO)��1 mol��L��1��cʼ(H2O)��1 mol��L��1��ijʱ�̾��ⶨCO��ת����Ϊ10%����÷�Ӧ________(�����Ѿ�������û����)�ﵽƽ�⣬��ʱ������ ______���� (����>������<������=��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����仯����㷺Ӧ���ڸ��²���������ش������й����⣺
��1��������Ľṹ��Ԫ������ʮ���壬ÿ����Ԫ����12����ԭ�ӣ���ͼ����
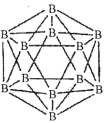
���ڻ�̬11Bԭ���У��������______�������෴�ĵ��ӡ�
����ÿ����Ԫ��������ԭ��Ϊl0B������Ϊ11B����õ�Ԫ�ṹ��������______�֡�
��2��NaBH4��һ����Ҫ�Ĵ������壬�����漰Ԫ�صĵ縺����С�����˳��Ϊ________��BH4-���ӵĿռ乹����_____________����BH4-��Ϊ�ȵ�����ĵķ�����___________��
��3������(H3BO3)Ϊ��ɫƬ״���壬����ʯī���ƵIJ�״�ṹ�������ᾧ���д��ڵ��������й��ۼ���_____��_______����H3BO3������ӽ�����__________������ĸ����
A.H4SiO4 B.H3PO4 C.HNO2
��4��������һ���ܵ��߶ȹ�ע����ĥͿ�ϣ��������������ı��汣���㡣��ͼ��������ľ�����Bԭ�ӵ��ӻ���ʽ��___________�������൪��������۵�Ҫ��������ߣ���ԭ����_____________��
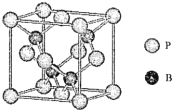
��5����֪����ľ�������a=478 pm������ʽ����þ�����ܶ�p=_____g��cm-3���ú�NA�Ĵ���ʽ��ʾ���ɣ�����Ҫ��������������������ԭ�Ӻ���ԭ������ĺ˼��Ϊ_________pm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A.���ӵ�����ԭ��ͨ��sp3�ӻ�����ɼ�ʱ���÷���һ��Ϊ��������ṹ
B.1,2�����ȱ���(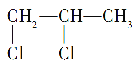 )�����к�����������̼ԭ��
)�����к�����������̼ԭ��
C.�ۡ��е㣺Na��Mg��Al
D.�������ȶ�������λ����ǿ���йأ�����λ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������̫���ܵ���е������ྦྷ��̫���ܵ�ء�GaAs̫���ܵ�ؼ�ͭ��������Ĥ̫���ܵ�صȡ���ش��������⣺
��1����̬��ԭ�ӵļ۵����Ų�ʽΪ___��H2O�ķе����H2Se�ķе�(��42��)����ԭ����___��
��2����֪GaCl3�����۵�Ϊ77.9�棬�е�Ϊ201.3�棬GaCl3Ʒ������Ϊ____��
��3��Na3AsO4�������ӵĿռ乹��Ϊ_____��Asԭ�Ӳ�ȡ_____�ӻ���
��4��������CuO����ת��ΪCu2O���Դ�ԭ�ӽṹ�ǶȽ���ԭ��_____��
��5��п��ͭλ��ͬһ���ڡ���п�ľ����ṹ��ͼ��ʾ��S2����Χ�Ⱦ����������Zn2+����Ϊ____���������߳�Ϊdpm������п���ܶ�Ϊ____g��cm��3(���ؼ�)��
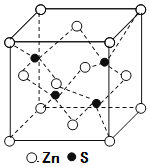
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ǵؿ��к����Ϸḻ��Ԫ�أ������������衢��Ԫ�أ��䵥�ʼ��Ͻ���������������Ӧ�ù㷺�Ľ������ϡ���ѧ�ϳ���KSCN��Һ����������Һ���Ƿ����Fe3+��
��1��Fe3+���ӻ�̬�ĵ����Ų�ʽ�ɱ�ʾΪ___��
��2��һ��������ľ����������������ѻ�����þ���������������ԭ�ӵĸ�����___��
��3��C��N����Ԫ�صļ���̬�⻯������ȶ�����ǿ������˳��Ϊ___��(�ѧʽ)
��4��C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ___(��Ԫ�ط���)��
��5�����ӻ�����Fe2O3���۵����KC1���۵��ԭ����___��
��6���ݱ�������Q��X��Z����Ԫ���γɵ�һ�־�����г����ԣ��侧��ṹ��ͼ��ʾ�������о�ÿ��Xԭ����Χ���������Q��ԭ�ӵĸ�����___��
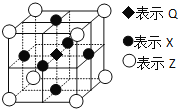
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ñ�Ũ���������Ƶ���Һ���ζ�δ֪Ũ�ȵ����ᣬ���в����л�ʹ����ⶨ��Ũ��ƫ����ǣ� ��
����ʽ�ζ���������ˮϴ����δ�ñ���Һ��ϴ
����ƿ��ʢ����������ˮ���ټӴ���Һ
����ʽ�ζ�������ˮϴ����δ��������ϴ
���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
���ζ���۲��ʽ�ζ��ܶ���ʱ�����ӿ̶���
A.�٢�B.�٢�C.�ڢ�D.�ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��ˮ��ȡþ����Ҫ�������£�
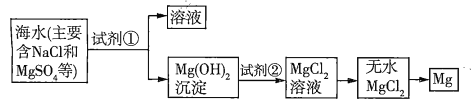
(1)���ڼ����Լ������������������·�����������������⡣
���� | �Ƿ���� | �������� |
���������¼���������ˮ���ټ�������� | a | b |
��������������������c |
a. _____________��
b.____________��
c.____________��
(2)��ͼ�м�����Լ���Ӧ����_______(����������)�������Լ��ڵ�������_______(�ѧʽ)����ҵ������ˮ![]() ��ȡþ�Ļ�ѧ����ʽΪ___________��
��ȡþ�Ļ�ѧ����ʽΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����H2 (g) + Se (g) ![]() H2Se (g) +87.48kJ ��Se (g) �� Se (s) +102.17kJ�� ����ѡ����ȷ����
H2Se (g) +87.48kJ ��Se (g) �� Se (s) +102.17kJ�� ����ѡ����ȷ����
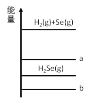
A.H2 (g) + Se (s)����������Ӧͼ���߶� b
B.��ͬ���ʵ����� Se��Se(s)���������� Se(g)
C.1mol Se (g)��ͨ�� 1mol H2(g)����Ӧ���� 87.48kJ
D.H2 (g) + S (g) ![]() H2S (g) +QkJ ��Q< 87.48kJ
H2S (g) +QkJ ��Q< 87.48kJ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com