£Ø2011?ÄĻ¾©ČżÄ££©īāĖįÄĘ¾§Ģå£ØNa
2MoO
4?2H
2O£©ŹĒĪŽ¹«ŗ¦ŠĶĄäČ“Ė®ĻµĶ³µÄ½šŹō»ŗŹ“¼Į£¬ÓÉīā¾«æó£ØÖ÷ŅŖ³É·ÖŹĒMoS
2£¬ŗ¬ÉŁĮæPbSµČ£©ÖʱøīāĖįÄĘ¾§ĢåµÄ²æ·ÖĮ÷³ĢČēĻĀ£ŗ
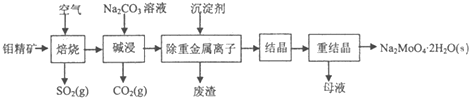
£Ø1£©Š“³ö”°¼ī½ž”±·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
MoO3+CO32-=MoO42-+CO2ӟ
MoO3+CO32-=MoO42-+CO2ӟ
£Ø2£©¼õ½žŅŗ½į¾§Ē°Šč¼ÓČėBa£ØOH£©z¹ĢĢåŅŌ³żČ„SO
42-£®µ±BaMoO
4æŖŹ¼³ĮµķŹ±£¬SO
42-µÄČ„³żĀŹŹĒ
97.3%
97.3%
£®[ŅŃÖŖ£ŗ¼ī½žŅŗÖŠc£ØMoO
42-£©=0.40mol?L
-1£¬c£ØSO
42-£©=0.04mol?L
-1£¬K
sp£ØBaSO
4£©=1.1”Į10
-10ӢK
sp£ØBaMoO
4£©=4.0”Į10
-8£¬¼ÓČėBa£ØOH£©
2¹ĢĢåŅżĘšµÄČÜŅŗĢå»ż±ä»ÆæÉŗöĀŌ£®]
£Ø3£©ÖŲ½į¾§µĆµ½µÄÄøŅŗæÉŅŌŌŚĻĀ“ĪÖŲ½į¾§Ź±ÖŲø“Ź¹ÓĆ£¬µ«“ļµ½Ņ»¶Ø“ĪŹżŗó±ŲŠė¾»»Æ“¦Ąķ£¬ŌŅņŹĒ
Ź¹ÓĆŅ»¶Ø“ĪŹżŗó£¬ÄøŅŗÖŠŌÓÖŹµÄÅضČŌö“ó£¬ÖŲ½į¾§Ź±»įĪö³öŌÓÖŹ£¬Ó°Ļģ²śĘ·“æ¶Č
Ź¹ÓĆŅ»¶Ø“ĪŹżŗó£¬ÄøŅŗÖŠŌÓÖŹµÄÅضČŌö“ó£¬ÖŲ½į¾§Ź±»įĪö³öŌÓÖŹ£¬Ó°Ļģ²śĘ·“æ¶Č
£®
£Ø4£©ČēĶ¼ŹĒĢ¼øÖŌŚ3ÖÖ²»Ķ¬½éÖŹÖŠµÄøÆŹ“ĖŁĀŹŹµŃé½į¹ū£ŗ
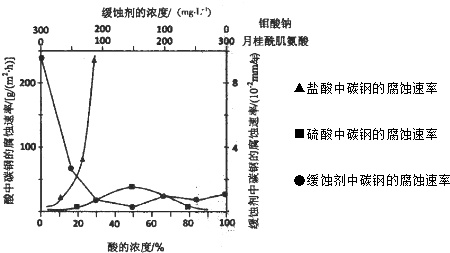
¢ŁĢ¼øÖŌŚŃĪĖįŗĶĮņĖįÖŠøÆŹ“ĖŁĀŹĖęĖįµÄÅØ¶Č±ä»ÆÓŠĆ÷ĻŌ²īŅģ£¬ĘäŌŅņæÉÄÜŹĒ
Cl-ÓŠĄūÓŚĢ¼øÖµÄøÆŹ“£¬SO42-²»ĄūÓŚĢ¼øÖµÄøÆŹ“£¬Ź¹µĆøÖĢśŌŚŃĪĖįÖŠµÄøÆŹ“ĖŁĀŹĆ÷ĻŌæģ
ÓŚĮņĖį£»ĮņĖįČÜŅŗĖę×ÅÅØ¶ČµÄŌö“ó£¬Ńõ±ČŠŌŌöĒ棬»įŹ¹øÖĢś¶Ū»Æ£¬øÆŹ“ĖŁĀŹ¼õĀż
Cl-ÓŠĄūÓŚĢ¼øÖµÄøÆŹ“£¬SO42-²»ĄūÓŚĢ¼øÖµÄøÆŹ“£¬Ź¹µĆøÖĢśŌŚŃĪĖįÖŠµÄøÆŹ“ĖŁĀŹĆ÷ĻŌæģ
ÓŚĮņĖį£»ĮņĖįČÜŅŗĖę×ÅÅØ¶ČµÄŌö“ó£¬Ńõ±ČŠŌŌöĒ棬»įŹ¹øÖĢś¶Ū»Æ£¬øÆŹ“ĖŁĀŹ¼õĀż
£®
¢ŚæÕĘųÖŠīāĖįŃĪ¶ŌĢ¼øֵĻŗŹ“ŌĄķŹĒŌŚøÖĢś±ķĆęŠĪ³ÉFeMoO
4-Fe
2O
3±£»¤Ä¤£®ĆÜ±ÕŹ½Ń»·ĄäČ“Ė®ĻµĶ³ÖŠµÄĢ¼øֹܵĄ»ŗŹ“£¬³żŠč¼ÓČėīāĖįŃĪĶā»¹Šč¼ÓČėNaNO
2£®NaNO
2µÄ×÷ÓĆŹĒ
Ģę“śæÕĘųÖŠŃõĘųĘšŃõ»Æ¼Į×÷ÓĆ
Ģę“śæÕĘųÖŠŃõĘųĘšŃõ»Æ¼Į×÷ÓĆ
¢ŪČō»ŗŹĶ¼ĮīāĖįÄĘ-ŌĀ¹šĖį¼”°±Ėį×ÜÅضČĪŖ300mg?L
-1£¬Ōņ»ŗŹ“Š§¹ū×īŗĆŹ±īāĖįÄʵÄĪļÖŹµÄĮæÅضČĪŖ
7.28”Įl0-4mol?L-1
7.28”Įl0-4mol?L-1
£®







 £Ø2011?ÄĻ¾©ČżÄ££©ŗĖÄÜŌ“ŅŃČÕŅę³ÉĪŖµ±½ńŹĄ½ēµÄÖ÷ŅŖÄÜŌ“£®
£Ø2011?ÄĻ¾©ČżÄ££©ŗĖÄÜŌ“ŅŃČÕŅę³ÉĪŖµ±½ńŹĄ½ēµÄÖ÷ŅŖÄÜŌ“£®