
�����Ǽ�����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͻ�������NO2���ܱ���������ˮ��η���ѭ�������Ʊ����ᡣ
��1����ҵ����ˮ����NO2����HNO3�����ɵ����徭������������յ�ѭ���������ת��Ϊ����(�ٶ�����������������ʧ)����д��������Ӧ�Ļ�ѧ����ʽ��
______________________________________________________________��
��2��Ϊ��֤��NOҲ������������ˮ��ͬ��Ӧ����HNO3��ijѧ���������ͼ��ʾװ��(�йؼг�װ������ȥ)��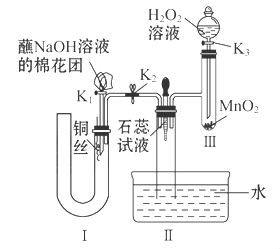
�ټ��װ�����������ú�Ϊ�۲쵽NO�������ɣ���K1���ر�K2��Ӧ��U�ιܵij��ܿ�ע��ϡ����������������Ѹ�ٹر�K1���۲쵽U�ι��ڵ�������____________________________________________________��
��װ�â��з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________��
��պNaOH��Һ�����ŵ�������___________________________________��
�ܴ�K2����װ�â��г��������е��������ɫ��K3����Ӧһ��ʱ����������в�δ����Һ�塣��Ƽ������鳤�������е������Ƿ�NO__________________________________________________��
��1��4NO2��O2��2H2O=4HNO3(��ֳ���������ʽд)
��2����U�ι��Ҳེ�����ء�U�ι����Һ������Ҷˣ�ͭ˿�����ܽ⣬������ɫ���壬��Һ����(���ٴ�����)����2H2O2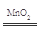 2H2O��O2��
2H2O��O2��
�����յ����������ֹ��Ⱦ����
���ٴ�K3�����۲쵽��������������Ѹ�ٱ�Ϊ����ɫ����֤��������NO��������ɫ�仯����֤������NO(����������Ҳ��)
�����������������ʵ��Ŀ�ĺ������õ��Լ������ƶϣ���װ�õ��������Ʊ�NO����װ�õ��������Ʊ�O2����װ�õ�������֤��NOҲ������������ˮ��ͬ��������HNO3��
��1��NO2�������������ˮ���յ�ѭ�����������ת��Ϊ����Ļ�ѧ����ʽΪ4NO2��O2��2H2O=4HNO3��
��2����ע��ϡ����Ӧ��U�ι��Ҳེ�����أ�Ѹ�ٹر�K1��U�ι����Һ������Ҷˣ�ͭ˿�����ܽ⣬������ɫ���壬��Һ������
��װ�â��з�����Ӧ�Ļ�ѧ����ʽΪ��2H2O2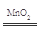 2H2O��O2����
2H2O��O2����
��պNaOH��Һ�����ŵ����������յ����������ֹ��Ⱦ������
���ٴ�K3�����۲쵽��������������Ѹ�ٱ�Ϊ����ɫ����֤��������NO��������ɫ�仯����֤������NO��
���㣺���⿼��ʵ��װ�ú�ʵ�鷽���ķ�������Ӧ�������������ѧ����ʽ����д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�١���ֱ�����йط�Ӧ�е�һ�����ʣ����Т�������ʹ��̪��Һ��죬���Ǻ���ɫ���壬�ش�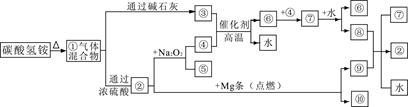
��1�����еĻ������ͨ��Ũ���ᷢ����ѧ��Ӧ����Ҫ������Ļ�ѧʽ�� ��
��2��д��̼������������ӵļ��鷽�� ��
��3��д����ҵ�Ϻϳɢ۵Ļ�ѧ����ʽ ��
��4������ᷴӦ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ڰ뵼�幤ҵ��������һ�仰������ɳ̲���û����������ɴֹ��ƴ���ij��÷���Ϊ��Si���֣�+2Cl2=SiCl4 SiCl4+2H2=Si������+4HCl������25��101KPa�����·�Ӧ����HCl����49L��ע��25��101KPa����������Ħ�����Ϊ24.5L/mol����
��1����Ӧ����HCl���������Ϊ__________��ת�Ƶ��ӵĸ���Ϊ_____________��
��2����Ӧ���ɵ�HCl��������127mLˮ�У��õ��ܶ�Ϊ1.20g/mL�����ᣬ����������ʵ���Ũ��Ϊ ��
��3������ɳ̲���û����漰�������Ӧ��������ȡ�ֹ�ķ�Ӧ����ʽΪ ��������ʯӢɰ���ռӦ�����Ƶ�ˮ��������Ӧ�����ӷ���ʽΪ ��
��4����ͨ����������������ʽ��ʾ�����Ϊ��������������Na2O 13����CaO 11.7����SiO2 75.3��������ʯ��ʯ�������ʯӢΪԭ���������ֲ���10t��ʯ��ʯ��������Ϊ80���������ʯӢ��������Ϊ95����������Ҫ����ԭ�ϵ������� t��������λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���绷�����˽���ȫ���ֹʹ��������������ˮ����������������ø�Ч����ɫ���������������ȡ�����������һ�ּ��ױ�ը��ǿ���������壬������ˮ�����ȶ����ʻ���ɫ����������ʹ��ʱ���뾡����ϡ���������ϡ�ͣ�ͬʱ��Ҫ������ա������ȡ�ʵ�����Ե�ⷨ�Ʊ�ClO2���������£�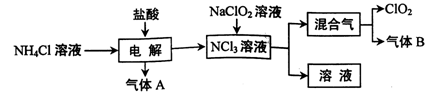
��1��ClO2������ԭ�� ����ǡ����ǡ���������8���ӽṹ����ͼ��ʾ�����ƵõĻ��������������B��ʹʯ����Һ����ɫ����ȥ�����������ѡ�� __��
A������ʳ��ˮ B����ʯ�� C��Ũ���� D������ˮ
��2���ȶ��Զ���������Ϊ�ƹ�������ȶ����������Ͳ�Ʒ������˵����ȷ���� ��
A���������ȿɹ㷺���ڹ�ҵ������ˮ����
B��Ӧ����ʳƷ��ҵ������Ч���ӳ�ʳƷ������
C���ȶ��Զ������ȵij��ִ�������˶������ȵ�ʹ�÷�Χ
D���ڹ������ͳ�Ʒ�������ڣ�Ҫ��ͨ��װ�úͼ�⼰����װ��
��3��ŷ������Ҫ��������������Ũ�����Ʊ������÷���ȱ���Dz��ʵ͡���Ʒ���Է��룬��������Ⱦ������д���÷��������Ļ�ѧ����ʽ ��
��4���ҹ��㷺���þ��������ϡ�͵�����������������ƣ�NaClO2����Ӧ�Ʊ�����ѧ����ʽ��
���˷����ŷ�������ŵ���
��5����ѧ�����о�����һ���µ��Ʊ����������������ữ�IJ��ᣨH2C2O4����Һ��ԭ�����ƣ���ѧ��Ӧ����ʽΪ ���˷���������������桢����İ�ȫ�ԣ�ԭ����____ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ������ũҵ�����о�����Ҫ�����ã���ϵ��ũҵ�����Ƿ���ա���ѧ������ũҵ�����еĹ㷺Ӧ�ã�Ϊ��ѧ���ϵĴ��ģ��ҵ�����ṩ����̨��
��1���ںϳɰ����豸(�ϳ���)�У������Ƚ�������Ŀ����______________��
��2����������Ĺ����г������һЩ���������һ��ɲ����������ַ���������
��Һ���շ���NO��NO2��2NaOH=2NaNO2��H2O
����ԭ����8NH3��6NO2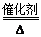 7N2��12H2O(NOҲ�����Ƶķ�Ӧ)
7N2��12H2O(NOҲ�����Ƶķ�Ӧ)
�����ɫ��ѧ�ĽǶȷ������ַ��������ӣ�____________________��
��3��ij���ʳ���NH3�Ʊ�NH4NO3����֪����NH3��NO�IJ�����96%��NO��HNO3�IJ�����92%������HNO3����ȥ��NH3������ռ�ܺ���NH3����(�������������)��________%��
��4���������һ�ֳ��õĵ��ʣ��������ʹ�øû���ʱ����Ӧע���������������±�(������Ӧע���������ɼ���)��
| | ע������ | ���� |
| �� | ______________ | ____________ |
| �� | ________________ | ____________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Na2SO3�dz��õĿ�������
��1��ʵ����ͨ����Ũ���ᣨ1��1����Na2SO3���Ʊ�SO2���壬
��Ӧ����ʽΪ�� ���Ʊ���SO2������ͨ������ˮ���������и�����ܸ���SO2������ǣ� ��
A.Ũ���� B.��ʯ�� C.��ˮCaCl2
��2�� ����SO2����ͨ��NaOH��Һ�пɵ�NaOH��Na2SO3�Ļ����Һ����û����Һ�м���������ˮ������Һ��Ϊ��ɫ��������Һ��Br2��Na2SO3����������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ______________��
��3����Ӧ�����Һ����SO32����SO42����Br����OH���������ӣ�����д��������SO32����SO42����Br����ʵ�鱨�棻
��ѡ�Լ���2 mol��L��1HCl��1 mol��L��1H2SO4��1mol��L��1HNO3��1 mol��L��1BaCl2��
1 mol��L��1Ba(NO3)2��0.1 mol��L��1AgNO3��CCl4���������Ʊ�����ˮ�����Ʊ�����ˮ��
| ��� | ʵ����� | Ԥ������ͽ��� |
| ����� | ȡ��������Һ���Թ�A�У��μ�2 mol��L��1HCl����Һ�����ԣ����뼸��________(���Լ�)���� | ________��֤������Һ�к�SO32- |
| ����� | ��ȡ��������Һ���Թ�B�У����� ���ٵμ����� 1 mol��L��1 BaCl2��Һ | |
| ����� | ��ȡ��������Һ���Թ�C�У� �������ú�۲���ɫ | ��Һ�ֲ㣬�ϲ�Һ��ʳȺ�ɫ��֤������Һ�к�Br- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������;ʮ�ֹ㷺�������ھ�ˮ�����������⣬�������������ᡢ��ȡ�
��1����ҵ�Ͽ���������ʯ����Ϊԭ������Ư�ۣ�Ư�۵���Ч�ɷ��� ��
��2����ҵ�������뵼����Ϲ���������£�
��д���Ʊ��ֹ�ʱ��Ӧ�Ļ�ѧ����ʽ�� ��
�ڴֹ���������Ӧ��õ��е�ϵ͵�Һ̬���Ȼ����г�����һЩ�߷е㡢�ѻӷ���Һ̬���ʣ�������з����ᴿ�����ᴿ����Ϊ ������ĸ����
| A������ | B������ | C����ȡ | D���ᾧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ֹ�������ˮ������ɱ����Ϊ���ƴ��ģ��Ⱦ�Լ�����������Ч����֮һ��Ư���dz��õ�������֮һ��
��1����ҵ�Ͻ�����ͨ��ʯ����[Ca(OH)2]��ȡƯ�ۣ���ѧ��Ӧ����ʽΪ ����Ӧ�٣���
��2��Ư������ˮ���ܿ����е�CO2���ã���������Ư�ס�ɱ�����õĴ����ᣬ��Ӧ��ѧ����ʽΪ ����Ӧ�ڣ���
��3��������ȶ������ֽ⣬��ѧ��Ӧ����ʽΪ ����Ӧ�ۣ���
��4����Ӧ�١���Ӧ�ڡ���Ӧ���У�����������ԭ��Ӧ���ǣ��Ӧ��ţ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
(8��) ���ֹ�������ˮ������ɱ����Ϊ���ƴ��ģ��Ⱦ�Լ�����������Ч����֮һ��Ư���dz��õ���������
��1����ҵ�Ͻ�����ͨ��ʯ����[Ca(OH)2]��ȡƯ�ۣ���ѧ��Ӧ����ʽΪ ��
��2��Ư�۵���Ч�ɷ��ǣ��ѧʽ�� ��
��3��Ư������ˮ���ܿ����е�CO2���ã�����Ư�ס�ɱ�������ܣ���ԭ���ǣ��û�ѧ��Ӧ����ʽ��ʾ�� ��
��4����Ӧ��1���ͷ�Ӧ��3���У�����������ԭ��Ӧ���ǣ����ţ� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com