
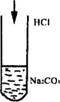
��ش�(1)�����������ֲ�ͬ�������̼�ʵ�����ݿ��жϼ���Һ��___________������Һ��___________��
(2)�����ӷ���ʽ��ʾ���β����õ���ͬ���������ԭ��
��_______________________________________________________��
��_______________________________________________________��
(3)����Һ�����ʵ���Ũ��Ϊ___________mol��L-1��(CO2��ˮ��Һ�е������ܽ���Բ���)
(4)��nmL�ļ���Һ������������Һ�����ֿ��ܵķ�ʽ��ϣ��������������ΪVmL(��״��)����V��ȡֵ��ΧΪ__________________��
(1)���� Na2CO3��Һ
(2)��2H++![]() ��CO2��+H2O
��CO2��+H2O
��H++![]() =
=![]()
![]() +H+====H2O+CO2��
+H+====H2O+CO2��
(3)0.8
(4)0��![]() ��8.96n
��8.96n
���������⿼��Na2CO3��HCl�������뷴��(�μ�˳��ͬ�����ﲻͬ)��������Σ���
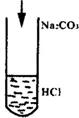
2HCl+ Na2CO3=2NaCl+H2O +CO2��
![]() 0.8mol/L��n��10-3L=0.4n��10-3mol
0.8mol/L��n��10-3L=0.4n��10-3mol
![]()
����Ƿ��Σ�Na2CO3+HCl=NaCl+NaHCO3
����Na2CO3����������CO2Ϊ��С��������![]() =0mol
=0mol
��V��ȡֵ��Χ:0��![]() ��8.96n
��8.96n


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ��Ŀ�� | ���鷽�� |
| �����Ȼ������Ƿ���� | D D |
| ��ȥʳ��������ϸɳ | C C |
| ��ȥ̼���ƹ���������̼������ | A A |
| ��ȥþ���л��е��������� | B B |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�Ƹ���ѧ����ʯ����2007�����������ѧ�Ծ� ���ͣ�022
| |||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ش�(1)�����������ֲ�ͬ�������̼�ʵ�����ݿ��жϼ���Һ��___________������Һ��___________��
(2)�����ӷ���ʽ��ʾ���β����õ���ͬ���������ԭ��
��_______________________________________________��
��_______________________________________________��
(3)����Һ�����ʵ���Ũ��Ϊ___________mol��L-1������Һ�����ʵ���Ũ��Ϊ_______mol��L-1(CO2��ˮ��Һ�е������ܽ���Բ���)��
(4)��n mL�ļ���Һ������������Һ�����ֿ��ܵķ�ʽ��ϣ������������壻��ΪV mL(��״��)����y��ȡֵ��ΧΪ___________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com