
ͭ�Ͻ�������ʹ������Ľ������ϣ�ͭ�ڻ������еij������ϼ���+l ��+2����֪Cu2O��ϡ���ᷴӦ���к�ɫ������������Һ����ɫ������Cu��Cu2O��CuO��ɵĻ�����У�����1 L 0.6 mol/L HNO3��Һǡ��ʹ������ܽ⣬ͬʱ�ռ���2240 mL NO���壨��״��������ش��������⣺
��1��д��Cu2O��ϡ���ᷴӦ�����ӷ���ʽ ��
��2�����������������������H2���Ȼ�ԭ�����õ����������Ϊ ��
��3����������к�0.1 mol Cu�����û������ϡ�����ַ�Ӧ����������H2SO4�����ʵ���Ϊ ��
��4�����������Cu�����ʵ���Ϊn mol����n��ȡֵ��ΧΪ ��
��1�� 3 Cu2O +14 H+ + 2 NO3- =" 6" Cu2+ + 2 NO�� + 7 H2O����2�֣�
��2�� 16 g ��2�֣���û��λ��1�֣�
��3��0.1 mol ��2�֣���û��λ��1�֣�
��4�� 0.05��n��0.15��2�֣�
���������������1���������ǿ�����ԣ���������ͭ��ͭ�Ļ��ϼ��ǣ�1�ۣ����л�ԭ�ԣ����Ը÷�Ӧ�����ӷ���ʽ��3 Cu2O +14 H+ + 2 NO3- =" 6" Cu2+ + 2 NO�� + 7 H2O��
��2����������ʵ�����0.6mol��������ԭ��������0.1mol�����Ը���ԭ���غ��֪����������ͭ�����ʵ�����0.25mol����ͭԭ�ӵ����ʵ�����0.25mol��������0.25mol��64g/mol��16g��
��3������Cu2O�����ʵ���Ϊm��CuO�����ʵ���Ϊn�������ɵ�ʧ�����غ�ɵ�0.1mol��2��2m��0.1mol��3�����m="0.05" mol����ͭԭ���غ�ɵ�n="0.05" mol�������Cu2O��H2SO4=CuSO4��Cu��H2O��CuO��H2SO4=CuSO4��H2O��֪������ϡ��������ʵ�����0.1mol��
��4���ɣ�2��֪ͭԪ�ص��ܵ����ʵ���Ϊ0.25 mol����Cu2O��CuO�����ʵ����ֱ�Ϊx��y�����ɵ�ʧ�����غ��2n��2x��0.1mol��3�����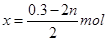 ����ͭ�غ��n��2x��y��0.25mol������y����n��0.05��mol����Cu2O��CuO�����ʵ����������㣬���Կɵ�n�ķ�ΧΪ0.05 mol��n��0.15 mol��
����ͭ�غ��n��2x��y��0.25mol������y����n��0.05��mol����Cu2O��CuO�����ʵ����������㣬���Կɵ�n�ķ�ΧΪ0.05 mol��n��0.15 mol��
���㣺����������ԭ��Ӧ���йؼ���
�������ڽ��л�ѧ����ʱ����Ҫȷ���շ�Ӧ�е��йػ�ѧ��Ӧԭ����д���йصĻ�ѧ����ʽ��Ȼ���ٽ����ڸ����غ㷨������ԭ���غ㡢�����غ㡢����غ㡢���ӵ�ʧ�غ�ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ͭ�Ͻ�������ʹ������Ľ������ϣ�ͭ�ڻ������еij������ϼ���+1��+2 �ȣ������γɶ���ͭ�Ļ����
ͭ�Ͻ�������ʹ������Ľ������ϣ�ͭ�ڻ������еij������ϼ���+1��+2 �ȣ������γɶ���ͭ�Ļ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 [��ѧ--���ʽṹ������]
[��ѧ--���ʽṹ������]�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�������
ͭ�Ͻ�������ʹ������Ľ������ϣ�ͭ�ڻ������еij������ϼ���+l ��+2����֪Cu2O��ϡ���ᷴӦ���к�ɫ������������Һ����ɫ������Cu��Cu2O��CuO��ɵĻ�����У�����1 L 0.6 mol/L HNO3��Һǡ��ʹ������ܽ⣬ͬʱ�ռ���2240 mL NO���壨��״��������ش��������⣺
��1��д��Cu2O��ϡ���ᷴӦ�����ӷ���ʽ ��
��2�����������������������H2���Ȼ�ԭ�����õ����������Ϊ ��
��3����������к�0.1 mol Cu�����û������ϡ�����ַ�Ӧ����������H2SO4�����ʵ���Ϊ ��
��4�����������Cu�����ʵ���Ϊn mol����n��ȡֵ��ΧΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�ϲ��и���������ģ��������ۻ�ѧ���� ���ͣ������
I��������Ա���֣�һЩ��ѧ��Ӧ�ڹ���֮�䷢������ˮ��Һ�з��������ﲻͬ��ͭ�Ͻ�������ʹ������Ľ������ϣ�ͭ�ڻ������еij������ϼ���+1��+2����CuCl2��2H2O�����NaOH��������ĥ������������һ��ɫ�Ĺ���M��M������ˮ����������ϡ����������ɫ��ҺB��
M�Ļ�ѧʽΪ ��M��ͬ��CuCl2��NaOH����Һ�з�Ӧ���ò���Ŀ���ԭ����
��
II��A+B��X+Y+H2O��δ��ƽ����Ӧ������ȥ������ѧ������Ӧ�Ļ�ѧ����ʽ������A��B�����ʵ���֮��Ϊ1��4����ش�
��1����Y�ǻ���ɫ���壬��Y�ĵ���ʽ�� ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��2����AΪ�������ʣ�������A��B��Ũ��Һ�С��ۻ�������A������X��Һ��
��AԪ�������ڱ��е�λ���� �����������ں��壩��Y�Ļ�ѧʽ�� ��
�ں�a mol X����Һ�ܽ���һ����A������Һ�����ֽ��������ӵ����ʵ���ǡ����ȣ���ԭ��X��____mol��
��3����A��B��X��Y��Ϊ�����A����ˮ������������Ӻ�ˮ���õ�������ɾ���ˮ����A��Һ�м��������ữ��AgNO33��Һ��������ɫ������B����ɫΪ��ɫ����A��B�����ʵ���֮��1��4ǡ�÷�Ӧ����Һ������Ũ�ȴӴ�С��˳���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com