
��֪AΪ����������X��YΪ�����ǽ�����X��E��F��G������Ϊ���壬CΪҺ
�壬B��һ���Σ����ȼ��ֽ⣬�ڹ�ũҵ��������;�Ϲ㣨�类����ijЩ��صĵ��
�ʣ�������A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й���֮���ת����
 ϵ����ͼ����ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ��
ϵ����ͼ����ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ��
��1����Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������䷴Ӧ����ʽΪ
�� ��
��2����D��Һ�Ʊ�D����ˮ����ġ�����a��Ϊ ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����������
��4����Ӧ�ݵĻ�ѧ����ʽΪ ����������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
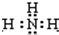
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
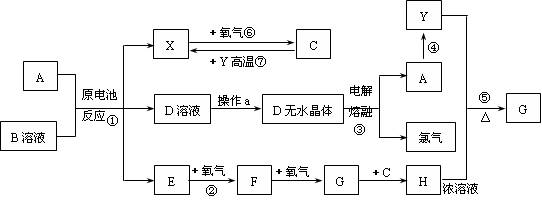
��֪AΪ����������X��YΪ�����ǽ�����X��E��F��G������Ϊ���壬CΪҺ�壬B��һ���Σ����ȼ��ֽ⣬�ڹ�ũҵ��������;�Ϲ㣨�类����ijЩ��صĵ���ʣ���H��Һ����ǿ�����ԡ�ǿ���ԡ�����A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й�����֮���ת����ϵ����ͼ��
��ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ��
����д���пհף�
��1����Ӧ��ΪA�ڶ�����̼��ȼ�գ����ɺ�ɫ���嵥��Y��A��������䷴Ӧ����ʽΪ
�� ��
��2����D��Һ�Ʊ�D����ˮ����ġ�����a��Ϊ ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ �������� ��
��4����Ӧ�ݵĻ�ѧ����ʽΪ ������ ����
��5��ԭ��ط�Ӧ���������ĵ缫��ӦʽΪ ��
��6��A��Ԫ�����ڱ�����λ���ǣ���_________���ڡ���__________�塣
��7��Y��ͬ����������__________��___________��____________�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ʡ����������ѧ���������νλ�ѧ�Ծ����������� ���ͣ������
��8�֣���֪AΪ����������X��YΪ�����ǽ�����X��E��F��G������Ϊ���壬CΪҺ�壬B��һ���Σ����ȼ��ֽ⣬�ڹ�ũҵ��������;�Ϲ㣨�类����ijЩ��صĵ���ʣ�������A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й�����֮���ת����ϵ����ͼ����ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ��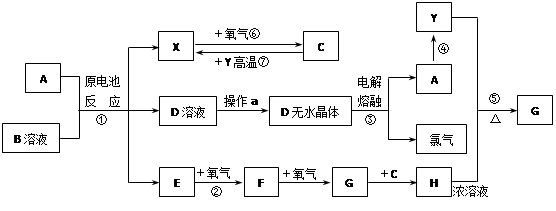
����д���пհף�
��1����Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������䷴Ӧ����ʽΪ ��
��2����D��Һ�Ʊ�D����ˮ����ġ�����a��Ϊ ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ����������
��4����Ӧ�ݵĻ�ѧ����ʽΪ ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡɽ���и���1���¿���ѧ�Ծ����������� ���ͣ������
��10�֣�����֪AΪ����������X��YΪ�����ǽ�����X��E��F��G������Ϊ���壬CΪҺ�壬B��һ���Σ����ȼ��ֽ⣬�ڹ�ũҵ��������;�Ϲ㣨�类����ijЩ��صĵ���ʣ�������A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й�����֮���ת����ϵ����ͼ��ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ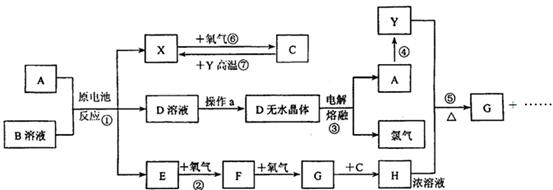
����д���пհף���1����Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������䷴Ӧ����ʽΪ ��
��2����D��Һ�Ʊ�D����ˮ����ġ�����a��Ϊ ��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ��
��4����Ӧ�ݵĻ�ѧ����ʽΪ ��
��5��ԭ��ط�Ӧ���������ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���㽭ʡ��У4�¸��������Ծ������ۣ���ѧ���� ���ͣ������
��֪AΪ����������X��YΪ�����ǽ�����X��E��F��G������Ϊ���壬CΪҺ�壬B��һ���Σ����ȼ��ֽ⣬�ڹ�ũҵ��������;�Ϲ㣨�类����ijЩ��صĵ���ʣ�������A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й�����֮���ת����ϵ����ͼ����ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ��
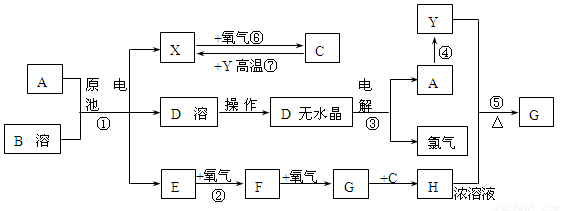
����д���пհף�
��1����Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������䷴Ӧ����ʽΪ
______________________________________________________________________��
��2����D��Һ�Ʊ�D����ˮ����ġ�����a��Ϊ____________________________��
��3��E�ĵ���ʽΪ_________________________________��
��4����Ӧ�ݵĻ�ѧ����ʽΪ_______________________________________________��
��5��ԭ��ط�Ӧ���������ĵ缫��ӦʽΪ___________________________________.
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com