
����Ŀ��T��ʱ����2L�����ܱ���������̬����X��Y��Z�����ʵ�����ʱ��仯�IJ������������ʾ��
T/min | n��X�� /mol | n��Y�� /mol | n��Z�� /mol |
0 | 0.80 | 1.00 | 0 |
1 | 0.70 | 0.80 | 0.20 |
5 | 0.70 | ||
9 | 0.40 | ||
10 | 0.40 | 0.80 |
��1��д���÷�Ӧ�Ļ�ѧ����ʽ_________________
��2����Ӧ���е�10min��X��ת����Ϊ___________��0~5min��Y��ƽ����Ӧ����Ϊ___________
��3������T��ʱ�÷�Ӧ��ƽ�ⳣ��K=____________
��4��T��������ܱ�������ͨ��һ������X��Y��Z����Ӧ��ijʱ�̲��X��Y��Z�����ʵ����ֱ�Ϊ1.00mol��0.50mol��1.60mol�����ʱ���淴Ӧ���ʴ�С��v�� v��������ڡ��������ڡ�����С�ڡ���
��5������10min��t3��t5ʱ�̷ֱ�ı�÷�Ӧ��ijһ��Ӧ�������õ�X��Z�����ʵ�������Ӧ������ʱ��Ĺ�ϵ����ͼA��B��ʾ��

10minʱ�ı����Ӧ������_______________
��t3ʱ�̸ı�ķ�Ӧ������_______________
��t5ʱ�̸ı�ķ�Ӧ������ ��������___________________
���𰸡���1��X+2Y![]() 2Z��2����
2Z��2����
��2��50%��1����0.07mol/��L��min����2����
��3��80L/mol��2������4��������2����
��5��������Y��Ũ����1�����ڽ��»��¶�
��ʹ�ô������ô�����Ӵ�����1�֣�����0����
t5ʱ�����淴Ӧ����ͬ�ȳ̶���������ȡ���2����
��������
�����������1���ɱ������ݿ�����Ӧ�ӿ�ʼ��ƽ�⣬X�����ʵ�����С��ӦΪ��Ӧ�0��1min���ʵ����仯ֵΪ1.00mol-0.90mol=0.10mol��Y�����ʵ�����С��ӦΪ��Ӧ�0��1min���ʵ����仯ֵΪ1.00mol-0.80mol=0.20mol��Z�����ʵ������࣬ӦΪ����������ʵ����ı仯ֵΪ0.20mol���������ʵ����ı仯�뻯ѧ�����������ȣ���n��X����n��Y����n��Z��=0.10mol��0.20mol��0.20mol=1��2��2�� X+2Y![]() 2Z����2����Ӧ���е�10min��X��ת������0.8-0.4��/0.8=0.5�� 0~5min��n��Z������0.7 mol��n��Y������0.7 mol��Y��ƽ����Ӧ����Ϊ0.7/2/5= 0.07mol/��L��min������3������ �������£�
2Z����2����Ӧ���е�10min��X��ת������0.8-0.4��/0.8=0.5�� 0~5min��n��Z������0.7 mol��n��Y������0.7 mol��Y��ƽ����Ӧ����Ϊ0.7/2/5= 0.07mol/��L��min������3������ �������£�
X+2Y![]() 2Z��
2Z��
��ʼ 0.80 1.00 0
�仯 0.40 0.80 0.80
ƽ�� 0.40 0.20 0.80
T��ʱ�÷�Ӧ��ƽ�ⳣ��K=��0.8/2��2/��0.4/2������0.2/2��2=80L/mol
��4������Ũ������ƽ�ⳣ���Ĺ�ϵ��Q>K,ƽ�����ƣ�Q��K,ƽ��������Q=K,ƽ�ⲻ�ƶ����������ݣ�Q=��1.6/2��2/��1/2������0.5/2��2=20.48 L/mol<80 L/mol,��Ӧ���ҽ��У�v������v��
��5����ͼA ������֪��������Z ��������Ӧ��X�������٣�ֻ��������Y��Ũ�ȣ�ƽ������������ͼB���Կ�����t3ʱ�̸ı������ȫ�����٣���v������v���� ֻ�ܽ��£����ʼ�����ƽ�����ƣ���t5ʱ�����淴Ӧ����ͻȻ��������ȣ��÷�Ӧ�����������Ӧǰ��Ϊ0����������ѹǿ��ֻ��ʹ�ô��� ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ݻ�������ܱ������г���һ����A��B���������·�Ӧ��xA��g����2B��s��![]() yC��g�� ��H<0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺
yC��g�� ��H<0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺
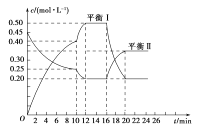
��1������ͼʾ��ȷ��x��y��________��
��2����A��Ũ�ȱ仯��ʾ�÷�Ӧ��0��10 min�ڵ�ƽ����Ӧ����v��A����______________��
��3��0��10 min������ѹǿ________�����������䡱��С������
��4���Ʋ��10 min�������߱仯�ķ�Ӧ����������____________����16 min�������߱仯�ķ�Ӧ����������____________��
����ѹ ������A��Ũ�� ������C���� ������ ������ ���Ӵ���
��5����ƽ������ƽ�ⳣ��ΪK1��ƽ����ƽ�ⳣ��ΪK2����K1____________K2�����������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�0.01 mol��L��1 MOH��Һ��pHΪ10��MOH��aq����H2SO4��aq����Ӧ����1 mol���εĦ�H����24.2 kJ��mol��1��ǿ����ǿ���ϡ��Һ���к���Ϊ��H����57.3 kJ��mol��1����MOH��ˮ��Һ�е���Ħ�HΪ
A����69.4 kJ��mol��1 B����45.2 kJ��mol��1 C����69.4 kJ��mol��1 D����45.2 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.78molFeCl2��Һ��ͨ��0.09molCl2�� �ټ���100mL1mol/L��X2O72-������Һ��ʹ��Һ�е�Fe2+ǡ��ȫ���������������X�Ļ��ϼ�Ϊ ( )
A��+3 B��+2 C��+1 D��+5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�ֱ���һ���Լ���ȥ���и������е�����(������Ϊ����)����д�����ӷ���ʽ��
��Mg(Al)
�Լ���________�����ӷ���ʽ��_____________________��
��CO2(HCl)
�Լ���________�����ӷ���ʽ��_____________________��
(2)���������ӷ���ʽ��д�ɻ�ѧ����ʽ��
��2H++CO![]() CO2��+H2O_____________________��
CO2��+H2O_____________________��
��Al3��+3OH��Al��OH��3��_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��N2��g��+3H2��g��![]() 2NH3��g�� ��H=-92.4KJ/mol����500�桢20MPaʱ����N2��H2ͨ�뵽���Ϊ2L���ܱ������У���Ӧ�����и������ʵ����ʵ����仯��ͼ��ʾ��
2NH3��g�� ��H=-92.4KJ/mol����500�桢20MPaʱ����N2��H2ͨ�뵽���Ϊ2L���ܱ������У���Ӧ�����и������ʵ����ʵ����仯��ͼ��ʾ��
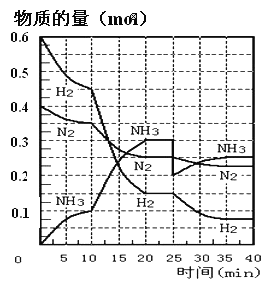
��1��10min����NH3��ʾ�÷�Ӧ��ƽ�����ʣ�v��NH3��= _______��
��2����10-20min��NH3Ũ�ȱ仯��ԭ�������_______������ĸ����
a.���˴��� b.�����¶� c.����NH3�����ʵ���
��3���ÿ��淴Ӧ�ﵽƽ��ı�־��___������ĸ��
a.3v��H2����=2v��NH3����
b.���������ܶȲ�����ʱ��仯
c.�����ڵ���ѹǿ������ʱ����仯
d.N2��H2��NH3�ķ�����֮��Ϊ1:3:2
e.��λʱ������mmolN2��ͬʱ����3mmolH2
f.amolN=N�����ѵ�ͬʱ����6amolN-M���ϳ�
��4����һ��ƽ��ʱ��ƽ�ⳣ��K1=____������ѧ����ʽ��ʾ����NH3�����������_____������2λС������
��5���ڷ�Ӧ���е�25minʱ�����߷����仯��ԭ����______��
��6����֪��N2��g��+3H2![]() 2NH3��g�� ��H=-92.4KJ/mol
2NH3��g�� ��H=-92.4KJ/mol
2H2��g��+O2��g��![]() 2H2O��g�� ��H=-483.6KJ/mol
2H2O��g�� ��H=-483.6KJ/mol
������ȫȼ��������̬ˮ���Ȼ�ѧ����ʽ��______
��7������ȼ�ϵ�ؾ��кܴ�ķ�չDZ��������ȼ�ϵ�ع���ԭ����ͼ��ʾ��
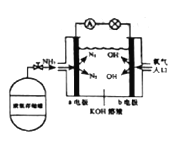
��b�缫�ĵ缫��Ӧʽ��_________��
��һ��ʱ�������װ���в���KOH�������ݷ�Ӧԭ������ԭ����_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л�������У���ij��̼ԭ���������ĸ���ͬ��ԭ�ӻ�ԭ����ʱ������̼ԭ�ӳ�Ϊ������̼ԭ���������磬��ͼ�л�������д���*��̼ԭ�Ӿ�������̼ԭ�ӡ����л���ֱ�������Ӧ�����ɵ��л���������Ժ�������̼ԭ�ӵ���

A. ������������H2��Ӧ
B. ��NaOHˮ��Һ��Ӧ
C. ����������Ӧ
D. �����ᷢ��������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС����һ�ֹ�ҵ�����Ʊ��ߴ�MgO��������������¡�

��ش��������⡣
��1�������������Mg2+�����ӷ���ʽΪ______________
��2���������ʿ����ڴ��沽�����H2O2����__________
A��NaCl B��Cl2 C������ D��KMnO4
��3��������в�����ͼ��ʾװ�ó�ȥFe3+

����ʵ��װ��ͼ������A������Ϊ________________
������������÷ֲ㣬������A�ϿڵIJ��������ϡ��²�������ȷ�����ǣ�________________
����ͼ�д��ڵĴ����ǣ�________________
��4���������NaOH���������______________ ������������������������
��5��ʵ���У�ȡ��ҵ����40.0g�� MgCO3�ĺ���Ϊ42%�����õ�4.0g�ߴ�����þ����ʵ���иߴ�����þ�IJ���Ϊ____________��![]() ��
��
��6���ߴ�����þ���ܾ����������ʺ���;����ȷ����____________
A������ B���ͼ� C�����ͻ����� D�����źŵ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ת��������û�л�ѧ�ܱ仯���ǣ� ��
A.�Ȼ���ȷֽⷴӦ
B.�����������������ɰ��ķ�Ӧ
C.������
D.��ɫֲ��Ĺ���������ɵ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com