
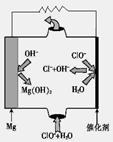
 ��
�� 
 CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H| �� �� | �� | �� |
| ��Ӧ�� Ͷ���� | 1molCO2 3molH2 | a molCO2��b molH2�� c molCH3OH(g)��c molH2O(g) |
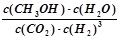 ��2�֣�
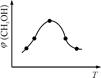
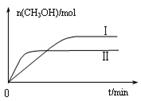
��2�֣� 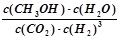 ������ͼ����֪����H<0���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ���¶����ߣ���Ӧ���ʺ��Ⱦ����������¶����ߵ�һ���̶ȣ���Ӧ�����ȷ�Ӧ���淴Ӧ���������Խ��Խ��ʹ�ü״������������(CH3OH)������С���۸���ͼ���֪��I�ļ״������ʵ�����ת���ʸߣ������ڼ���ʱ��ƽ�ⳣ���Ĺ�ʽ��
������ͼ����֪����H<0���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ���¶����ߣ���Ӧ���ʺ��Ⱦ����������¶����ߵ�һ���̶ȣ���Ӧ�����ȷ�Ӧ���淴Ӧ���������Խ��Խ��ʹ�ü״������������(CH3OH)������С���۸���ͼ���֪��I�ļ״������ʵ�����ת���ʸߣ������ڼ���ʱ��ƽ�ⳣ���Ĺ�ʽ��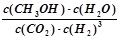 ��ƽ�ⳣ����С��ϵΪK��>K�����ܸ��ݻ�ѧƽ�ⷴӦ���м������£�
��ƽ�ⳣ����С��ϵΪK��>K�����ܸ��ݻ�ѧƽ�ⷴӦ���м������£� CH3OH(g) +H2O(g)
CH3OH(g) +H2O(g)


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
 ���ڣ��Ҳ���Hԭ��ֱ��������ȼ�ϵ���������N2O4��ȼ�ղ���ֻ��CO2��H2O��N2��5.00g��ƫ�����¡���ȫȼ��ʱ�ɷų�212.5kJ������
���ڣ��Ҳ���Hԭ��ֱ��������ȼ�ϵ���������N2O4��ȼ�ղ���ֻ��CO2��H2O��N2��5.00g��ƫ�����¡���ȫȼ��ʱ�ɷų�212.5kJ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)===H2O(l)����H3����285.8 kJ/mol
O2(g)===H2O(l)����H3����285.8 kJ/mol| A��488.3 kJ/mol | B����224.15 kJ/mol |
| C����488.3 kJ/mol | D��244.15 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����44.2 kJ��mol-1 | B��+44.2 kJ��mol-1 |
| C����330 kJ��mol-1 | D��+330 kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 ��
�� 

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ȼ����Ϊ-285.5kJ��mo1����ˮ�����Ȼ�ѧ����ʽΪ�� 2H2O��1�� =2H2��g��+O2��g������H=+285.5KJ��mo1 |
| B��1mol������ȫȼ������CO2��H2O��1��ʱ�ų�890kJ�����������Ȼ�ѧ����ʽΪ 1/2CH4��g��+O2��g��= 1/2CO2��g��+H2O��1������H= һ445kJ��mol |
| C����֪2C��s��+O2��g��=2CO��g������H= һ221kJ��mol-1����C��ȼ����Ϊһ110.5kJ��mo1 |
| D��HF��NaOH��Һ��Ӧ��H+��aq��+OH����aq��=H2O��1������H= һ57.3kJ��mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com