
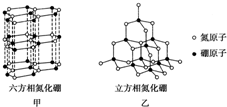
 ������BN�������ж�����ṹ�������൪������ͨ�����ڵ��ȶ��࣬��ʯī���ƣ�ֻ�в�״�ṹ�������������������൪�����dz�Ӳ���ϣ����������ĥ�ԣ����ǵľ���ṹ��ͼ��ʾ��
������BN�������ж�����ṹ�������൪������ͨ�����ڵ��ȶ��࣬��ʯī���ƣ�ֻ�в�״�ṹ�������������������൪�����dz�Ӳ���ϣ����������ĥ�ԣ����ǵľ���ṹ��ͼ��ʾ������ ��1��Bԭ�Ӻ��������Ϊ5�������������ԭ����д��������Ų�ʽ��
��2����a�������൪������Nԭ����Bԭ��֮���γɵ�����
b�������൪������������Ϊ���»�����
c�����־����е�B-N����Ϊ���ۼ���
d�������൪�����dz�Ӳ���ϣ����������ĥ�ԣ�����ԭ�Ӿ��壻
e�������൪����ľ�������ʯ���ƣ������к���8��ԭ�ӣ�B��Nԭ����Ŀ֮��Ϊ1��1��
��3�������൪���������һ����ԭ�������ڵ�ԭ�ӹ���ƽ�������Σ������൪������IJ�״�ṹ��û�����ɵ��ӣ�
��4�������൪�������У���ԭ���γ�4���Ҽ���û�йµ��Ӷԣ��ڵؿ��ڲ��������Խ���ѹǿԽ���¶�Խ�ߣ�
��5��һ��NH4BF4��Nԭ�Ӻ�����һ��Hԭ��֮�������λ����Bԭ�Ӻ�����һ��Fԭ��֮�����һ����λ�������Ժ���2����λ����
��� �⣺��1����̬��ԭ�Ӻ�����5�����ӣ��ֱ�λ��1s��2s��2p�ܼ������ݹ���ԭ��֪���̬�ĵ����Ų�ʽ1s22s22p1���ʴ�Ϊ��1s22s22p1��
��2a�������൪������Nԭ����Bԭ��֮���γɵ����������м�������ԭ�Ӿ��壬����Ӳ�ȴ�a����
b�������൪������������Ϊ���»����������ʵ�������b��ȷ��
c�����־����е�B-N����Ϊ���ۼ�����c��ȷ��
d�������൪�����dz�Ӳ���ϣ����������ĥ�ԣ�����ԭ�Ӿ��壬��d����
e�������൪����ľ�������ʯ���ƣ������к���8��ԭ�ӣ�B��Nԭ����Ŀ֮��Ϊ1��1������4��Bԭ�ӣ�4��Nԭ�ӣ���e��ȷ��
��ѡ��bce��
��3�������൪���������һ����ԭ�������ڵ�ԭ���γ�3�����۵�������Bԭ�Ӳ����ڹµ��Ӷԣ����Թ��ɵĿռ乹��Ϊƽ�������Σ������ʵIJ�״�ṹ�в����������ƶ��ĵ��ӣ����Բ����磬
�ʴ�Ϊ��ƽ�������Σ���״�ṹ��û�������ƶ��ĵ��ӣ�
��4�������൪�������У���ԭ���γ�4���Ҽ���û�йµ��Ӷԣ��ӻ������ĿΪ4����ȡsp3�ӻ���
�����൪�������У���ԭ�Ӻ��ĸ�Nԭ���γ�4�����۵���������Bԭ�ӵ��ӻ��������Ϊsp3���ڵؿ��ڲ��������Խ���ѹǿԽ���¶�Խ�ߣ��������֪��ʵ�����������൪����ϳ������൪������Ҫ������Ӧ�Ǹ��¸�ѹ��
�ʴ�Ϊ��sp3�����¸�ѹ��
��5��һ��NH4BF4��Nԭ�Ӻ�����һ��Hԭ��֮�������λ����Bԭ�Ӻ�����һ��Fԭ��֮�����һ����λ�������Ժ���2����λ������1mol NH4BF4����2mol��λ����
�ʴ�Ϊ��2��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų����ӻ���������ӽṹ�������ṹ����λ���ȣ�ע������������ɵ��ӷ��������൪�����岻���磬�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cr2O3 | B�� | MnO2 | C�� | WO3 | D�� | MgO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 58.5gNaCl�ܽ���1Lˮ����ɵ���Һ������Һ�����ʵ���Ũ��Ϊ1mol/L | |
| B�� | ��22.4L��HCl��������ˮ���Ƴ�1L��Һ����Ũ��Ϊ1mol/L | |
| C�� | ����480ml 1.0mol/L��NaOH ��Һ��ҪNaOH ����20.0g | |
| D�� | �к�100ml 1 1mol/L ��H2SO4��Һ����ҪNaOH 4g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 100 mL 0.1 mol/L��Na2CO3��Һ�м���0.01 molCH3COOH��CO32-+CH3COOH�THCO3-+CH3COO- | |
| B�� | ��HCOOK��KOH�Ļ����Һ�м���KMnO42KMnO4+HCOOK+KOH�T2K2MnO4+CO2��+H2O | |
| C�� | 4 mol/L��NaAlO2��Һ��7 mol/L�������������Ȼ�ϣ�4AlO2-+7H++H2O�T3Al��OH��3+Al3+ | |
| D�� | �ں���Mn2+����Һ�м���HNO3�ټ���PbO2����Ӧ��ϵ���Ϻ�ɫ��5PbO2+2Mn2++4H+�T5Pb2++2MnO4-+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 6 | B�� | 3 | C�� | 5 | D�� | 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 22.4 L NH3�к���ԭ����Ϊ NA | |
| B�� | 1 mol Na2O2��ˮ��ȫ��Ӧʱת�Ƶĵ�����ΪNA | |
| C�� | 100 mL 2.0 mol•L-1 NH4HCO3��Һ��NH4+��Ϊ0.2NA | |
| D�� | 1 mol O2��2 mol SO2���ܱ������г�ַ�Ӧ��ķ���������2NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com