
�����£������100mLˮ�ĸ��������зֱ����0.10molþ�ۡ�0.50mol���ۼ���ͬ���ʵ������Ȼ��Ʒ�ĩ�����Ͻ��裬ʵ��ʱ�ֱ��¼��Ӧ������0-15minʱ�¶����ߵķ��ȣ��μ���ͼ��������˵���������
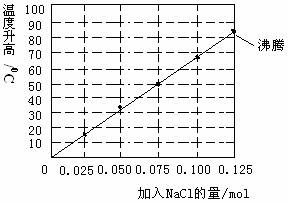
A��NaCl���������ش���0.125molԭ�����ټ���NaCl�����ή�ͷ�Ӧ����
B�������ʵ���м�����0.060molNaCl�����15minʱ�������¶Ƚӽ���42��
C�����ۡ�NaCl��ʹ��Ӧ�������ӣ�ԭ����þ�ۡ�������NaCl��ˮ��Һ������ԭ���
D�����������������£������0.10molþ�۸ijɵ�����þ����������0.075molNaClʱ����������ֵ��С��50

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڸ��������£����мӵ�������ڻ�ѧ��Ӧ����ȫ���ĵ���
A����״���£���1 g��ƬͶ��20 mL 18.4 mol��L��1��������
B�������£���100 mL 3 mol��L��1�������м���6.4 gͭ
C�����ʵ��¶Ⱥʹ��������£���2 mol SO2��1 mol O2�ϳ�SO3
D������������H2O(g)��H2ͨ��ʢ������Na2O2�����в������õ��ȼ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڸ��������£����мӵ�������ڻ�ѧ��Ӧ����ȫ���ĵ���
A����״���£���1 g��ƬͶ��20 mL 18.4 mol��L��1��������
B�������£���100 mL 3 mol��L��1�������м���6.4 gͭ
C�����ʵ��¶Ⱥʹ��������£���2 mol SO2��1 mol O2�ϳ�SO3
D������������H2O(g)��H2ͨ��ʢ������Na2O2�����в������õ��ȼ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ�����и�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
I����ѧ��һ����ʵ��Ϊ���Ŀ�ѧ����ѧʵ����ѧϰ̽���������ʵĻ�������֮һ����1����ѧʵ���У�������Һ���Լ������ữ�������ữ�����Ĵ�ʩ����ȷ����
A�����Լ���SO32-������HNO3�ữ��BaCl2��Һ
B������FeCl2��Һʱͨ��������HNO3�ữ����С��ˮ��̶�
C������ij��Һ���Ƿ�Cl-����HNO3�ữ��AgNO3��Һ
D�����Ը��������Һ�����������ữ
��2�������й�˵������ȷ����??????? ?
����pH��ֽ�����ˮ��pHΪ3��5
��������������NaOH����
������ŨNaOH��Һ�����Ȳ���ʹʪ��ĺ�ɫʯ����ֽ���������壬��ԭ��Һ��һ������NH4+
������Һ�м�������˫��ˮ���ټӼ���KSCN��Һ����Һ��죬��ԭ��Һ��һ������Fe2+
��ʵ��ʱ����������������ָ����������Ѫʱ���������Ȼ�����ҺͿĨֹѪ
II����1��ij�¶��£���ˮ��c(H+)=2��0��10-7mol��L-1�����¶��£�0��9mol��L-1NaOH��Һ��0��1mol��L-1 HCl��Һ��������(��������Һ����仯)������Һ��pH= ???? ��
��2������0��020 molCH3COOH����Һ�м���0��020mol CH3COONa���壬��ҺpH������Ҫԭ���� ?? ?? ����֪�û����Һ��c(Na+)<c(CH3COO-)����c(CH3COOH)?????? c(CH3COO-)(����>������<������=���������)��
��3�������£���100 mL 0��01mol��L-1HA��Һ��μ���0��02mol��L-1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���(����仯���Բ���)��
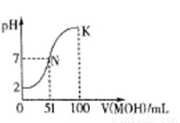
�ش��������⣺
����ͼ����Ϣ��֪HAΪ??? ��(����ǿ����������)��
��K���Ӧ����Һ�У�c(M+)+c(MOH)=???? mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡТ�������꼶��һ��ͳһ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���жԻ�ѧʵ������Ľ��ͻ������ȷ����
ѡ�� ��ʵ ���ͻ����
A �����£���Na2CO3��Һ�м�������BaSO4��ĩ�����ˣ���ϴ���ij����м�ϡ���ᣬ�������� �����£�
Ksp(BaCO3)>Ksp(BaSO4)
B �������������Ʒ�Ӧ������ʹƷ����Һ��ɫ������ �ǽ����ԣ�Cl> S
C ���з�̪��NaHCO3��Һ��dz��ɫ���Ⱥ��ɫ���� NaHCO3�ֽ�������Na2CO3��������ǿ
D ����ͨ��Al(OH)3����ʱ�ᷢ�������ЧӦ �������ӵ�ֱ��Ϊ1nm~100 nm
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��³����1��3����Ȼ���е�Ԫ����ϰ ���ͣ�ѡ����
�ڸ��������£����мӵ�������ڻ�ѧ��Ӧ����ȫ���ĵ���
A����״���£���1 g��ƬͶ��20 mL 18.4 mol��L��1��������
B�������£���100 mL 3 mol��L��1�������м���6.4 gͭ
C�����ʵ��¶Ⱥʹ��������£���2 mol SO2��1 mol O2�ϳ�SO3
D������������H2O(g)��H2ͨ��ʢ������Na2O2�����в������õ��ȼ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com