
 4H++2Br-+S
4H++2Br-+S


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
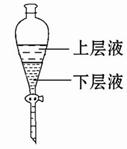
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
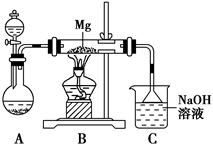
 2MgSO3��S����ͬѧ���Ʋ��ǣ�3Mg��SO2
2MgSO3��S����ͬѧ���Ʋ��ǣ�3Mg��SO2 2MgO��MgS��Ҫ��֤�ס��ҡ�����λͬѧ���Ʋ��Ƿ���ȷ����ͬѧ������ʵ��̽����
2MgO��MgS��Ҫ��֤�ס��ҡ�����λͬѧ���Ʋ��Ƿ���ȷ����ͬѧ������ʵ��̽����| ��� | ʵ�鲽�� | Ԥ������ͽ��� |
| �� | ȡ������Ӧ�����ù������Թ��� | |
| �� | ���Թ��еĹ��������μ�____________���Թܿ����ϴ����ܵĵ���������������ͨ��ʢ��________���Թ��� | ���Թ��е�________�����ͬѧ�Ʋ���ȷ�����Թ��еĹ���δ��ȫ�ܽ⣬��________������ͬѧ�Ʋ���ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Fe3+ | B��Ba2+ | C��Cu2+ | D��H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
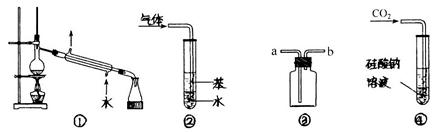
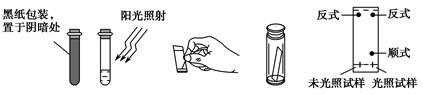
| A��װ�â٣������ڷ���ʯ�ͣ��ֱ�õ����͡�ú�ͺͲ��͵ȸ��ִ����� |
B��װ�âڣ����������� ���壬����ֹ���� ���壬����ֹ���� |
C��װ�âۣ������a��b�����������ռ�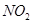 �������b��a�����������ռ� �������b��a�����������ռ�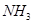 |
D��װ�âܣ�����ͨ��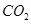 ���壬�������ȳ��ְ�ɫ������������ ���壬�������ȳ��ְ�ɫ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������Һ�еμ��������ʹ����ʯ��ˮ����ǵ����壬��Һ�в�һ������CO32- |
| B���μ�AgNO3��Һ������ɫ��������Һһ����Cl�� |
| C������Һ�м��������ữ��BaCl2��Һ������ɫ��������ԭ��Һ�к�SO42- |
| D������Һ�еμ���ˮ��KSCN����Һ��죬����Һ��һ����Fe3�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com