
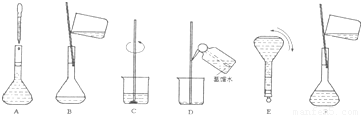
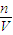 �жϲ�����������ҺŨ�ȵ�Ӱ�죮
�жϲ�����������ҺŨ�ȵ�Ӱ�죮 ������Һ����������������
������Һ���������������� 

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�꽭��ʡ������ʮ���ظ�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
ʵ��������CuSO4��5H2O������480mL0.1mol/L��CuSO4��Һ����ش��������⣺
��1��Ӧ��������ƽ��ȡCuSO4��5H2O����_______g��
��2�����ڳ�����Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ�1g����ʹ�����룩����ƽƽ��ʱ��ʵ�ʳ�����CuSO4��5H2O������__________g��
��3����ʵ���õ�����Ҫ�����У�������ƽ����Ͳ���ձ�����������________��________��
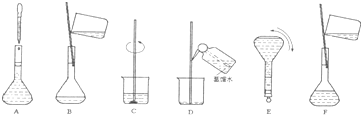
��4�����ƹ������м����ؼ��IJ���Ͳ�������ͼ��ʾ������Щʵ�鲽��A��F��ʵ������Ⱥ������
�� ��
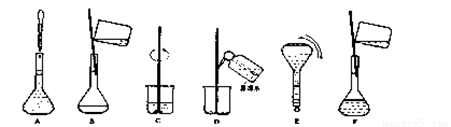
���ж��ݵľ�������� ��
��5�����������ʹ������Һ��Ũ�Ȳ�������Ӱ�죨A.ƫ�� B.ƫ�� C.����,����š�����
���ܽ⾧���õ��ձ��Ͳ�����δϴ�ӣ�____________��
�ڶ���ʱ���ӿ̶��ߣ�____________��
������CuSO4��5H2O������ʧȥ���ֽᾧˮ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com