
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ˮ��Һ�Ⱦ�ˮ��ԭ����ͬ |
| B���Ʋ������ͭ����ȶ�п�������ʴ |
| C����Ҫʱ����в����⻯�������ɴﵽ�˹������Ŀ�� |
| D��̼���ƿ����ڱ��Ƹ�㣬Ҳ����������θ�����֢ |
�鿴�𰸺ͽ���>>
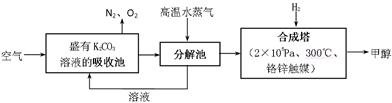
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
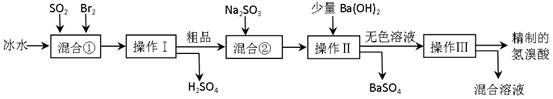
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
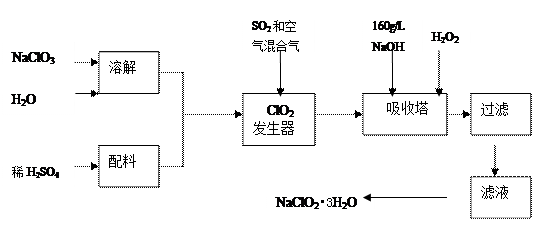

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3��g������
2NH3��g������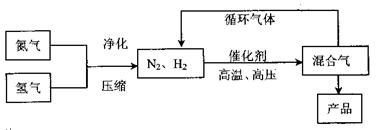
 2NH3��g���ġ�H= ��
2NH3��g���ġ�H= ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���⣨������ | B���壨��ˮɹ�κ����Һ�� |
| C����ϩ���Ҵ��� | D��˳����ʯ���ѽ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
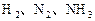 ��Ӧ��ƽ��ʱ������Ũ������һ���ǣ� ��
��Ӧ��ƽ��ʱ������Ũ������һ���ǣ� ��| | 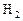 | 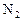 | 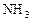 |
| A | 6 | 2 | 0 |
| B | 1 | 0 | 4 |
| C | 3.5 | 1 | 2 |
| D | 5 | 1.5 | 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ͨ�����ʯ��ˮ��ȡƯ�� |
| B����ⱥ��ʳ��ˮ��Һ��ȡ������ |
| C��SO2����ΪSO3ʱ��Ҫʹ�ô����������������SO2��ת���� |
| D����98.3%��ŨH2SO4����SO3��Ŀ���Ƿ�ֹ�γ�������ʹSO3������ȫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ú���͵ķ��� | B��ú�ĸ��� | C��ú������ | D��ú��Һ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com