
| ���� | ������ƽ�������룩 | С�ձ� | ����ƿ | ������ | ҩ�� | ��Ͳ | ��ͷ�ι� |
| ���� |  |
 |
 |
 |
 |
 |
 |
| ��� | a | b | c | d | e | f | g |

| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1000��1.8��98% |
| 98 |
| 1.337KJ |
| 0.025mol |


 �߽�������ϵ�д�
�߽�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������Fe������������Ӧ�������� |
| B���������������Сһ�룬������Ӧ���������淴Ӧ���ʼ�С |
| C������������䣬����ˮ����ʹ��ϵѹǿ����������Ӧ���������淴Ӧ���ʼ�С |
| D������ѹǿ���䣬����N2ʹ�������������������Ӧ���ʼ�С���淴Ӧ����Ҳ��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
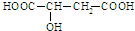 �������й�ƻ�����˵������ȷ���ǣ�������
�������й�ƻ�����˵������ȷ���ǣ�������| A��1molƻ�������������Ʒ�Ӧ����������3g |
| B��1molƻ��������������������Һ��Ӧ������3mol�������� |
| C��1molƻ��������������̼��������Һ��Ӧ�����������44.8L�Ķ�����̼���� |
| D��ƻ������һ�������¼�������ᷴӦ���������Ҵ���Ӧ��Ҳ������������������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��25��ʱ����0.1mol?L-1 NH4HSO4��Һ�еμ�NaOH��Һ�����ԣ�c��Na+����c��NH4+����c��SO42-����c��OH-��=c��H+�� |
| B��25��ʱ��NaB��Һ��pH=8��c��Na+��+c��B-��=9.9��10-7mol?L-1 |
| C��0.1mol?L-1��NaHCO3��Һ�У�c��OH-��+c��CO32-��=c��H+��+c��H2CO3�� |
| D��ͬ���£�pH��ͬʱ����Һ���ʵ���Ũ�ȣ�c��CH3COONa����c��NaHCO3����c��C6H5ONa����c��Na2CO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��F��N��O |
| B��O��Cl��F |
| C��As��P��H |
| D��Cl��S��As |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
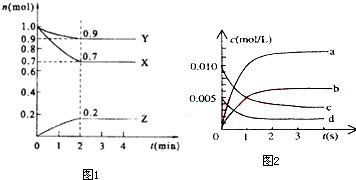
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����t��ʱ��ijNaOHϡ��Һ�У�c��H+��=10-amol/L��c��OH-��=10-bmol/L����֪a+b=12����
����t��ʱ��ijNaOHϡ��Һ�У�c��H+��=10-amol/L��c��OH-��=10-bmol/L����֪a+b=12�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
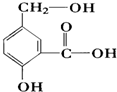 ��ij�л���A�ķ�����ṹ��ʽ��ͼ����ش�
��ij�л���A�ķ�����ṹ��ʽ��ͼ����ش��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com