
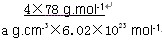 ��3�֣� ��5��12��2�֣�
��3�֣� ��5��12��2�֣�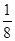 +6��
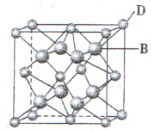
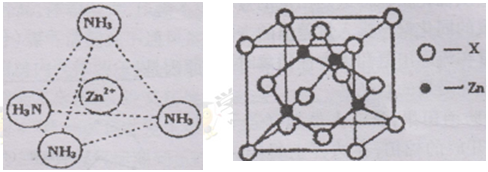
+6��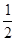 =4��Fԭ�Ӵ��ھ����ڲ����������з�ԭ����ĿΪ8����ΪDF2��DΪ+2�ۣ�D������Ԫ�أ����븱��Ԫ��Eͬ���ڣ���DΪCaԪ�أ�
=4��Fԭ�Ӵ��ھ����ڲ����������з�ԭ����ĿΪ8����ΪDF2��DΪ+2�ۣ�D������Ԫ�أ����븱��Ԫ��Eͬ���ڣ���DΪCaԪ�أ�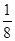 +6��
+6��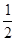 =4��Fԭ�Ӵ��ھ����ڲ����������з�ԭ����ĿΪ8����ΪCaF2������������Ϊ
=4��Fԭ�Ӵ��ھ����ڲ����������з�ԭ����ĿΪ8����ΪCaF2������������Ϊ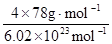 ��������ܶ�Ϊ�ѣ�g?cm-3�������������
��������ܶ�Ϊ�ѣ�g?cm-3�������������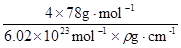 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A���ø������д��ڻ�ѧ������ֻ�����Ӽ�����λ�� |
| B���ø�������Clԭ�ӵ��ӻ�����Ϊsp3 |
| C���ø�������ֻ��CO��H2O��Ϊ��λ�� |
| D��CO��N2�ļ۵���������ͬ����ṹΪC=O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A�����Ǿ��ɼ���ȥ��һ����ԭ������ |
| B�����ǻ�Ϊ�ȵ����壬̼ԭ�Ӿ���ȡsp2�ӻ� |
| C��CH3-��NH3��H2O+��Ϊ�ȵ����壬���ι��;�Ϊ������ |
| D��CH3+�е�̼ԭ�Ӳ�ȡsp2�ӻ�������ԭ�Ӿ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������з����� | B�����еĻ�ѧ��ǿ�ȸ�ǿ |
| C�������Ӽ���������С | D����̬ʱ���������и����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 A��N2����3CO2��(����ƽ)
A��N2����3CO2��(����ƽ)�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com