
(ÿ��1�� ��6��) һ���ѧ�о��ɹ�������ͭ��������(CuMn2O4)���ڳ����´����������е�һ����̼�ͼ�ȩ(HCHO)��
(1) ��һ�����ʵ���Ũ�ȵ�Cu(NO3)2��Mn(NO3)2��Һ�м���Na2CO3��Һ�����ó������������գ����Ƶ�CuMn2O4��
�� Mn2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ ��
�� NO3���Ŀռ乹��Ϊ (����������)��
(2) ��ͭ��������Ĵ��£�CO��������CO2��HCHO��������CO2��H2O��
�� ���ݵȵ���ԭ����CO���ӵĽṹʽΪ ��
�� H2O������Oԭ�ӹ�����ӻ�����Ϊ ��
�� 1molCO2�к��еĦҼ���ĿΪ ��
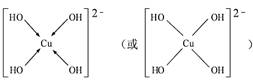
(3) ��CuSO4��Һ�м������NaOH��Һ������[Cu(OH)4]2���������ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ ��
(ÿ��1�֣���6��) (1) ��1s22s22p63s23p63d5(��[Ar]3d5) �� ƽ��������
(2) ��C��O �� sp3 �� 2��6.02��1023��
(3) 
����������1���ٿ��������ӵ��ձ���ɡ����ݹ���ԭ����֪�� Mn2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ1s22s22p63s23p63d5(��[Ar]3d5) ��
�ڸ��ݼ۲���ӶԻ������ۿ�֪��NO3���е�ԭ�Ӻ��еŶԵ��Ӷ����ǣ�5��1��2��3����2��0�����Ը����ӵĽṹ��ƽ�������νṹ��
��2���۵�������ԭ�����ֱ���ȵ��ǵȵ����壬���CO�͵�����Ϊ�ȵ����塣���������к������������CO�Ľṹʽ��C��O��
��ˮ������V�νṹ������ԭ�Ӻ���2�Թ¶Ե��ӣ��������ӻ����������sp3�ӻ���
������˫��������1���Ҽ���1���� �����ɵģ����Ը���CO2�ĽṹʽO��C��O��֪�������к���2���Ҽ���1molCO2�к��еĦҼ���ĿΪ2��6.02��1023����
��3��[Cu(OH)4]2��������λ���γɵ����ӣ�����OH�������壬ͭ�����ṩ�չ����������ṹʾ��ͼ��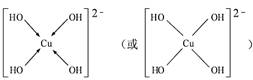 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(ÿ��1��,��6��)�������б仯�����ƶϡ�
\x(����B����HClð���̰�ɫ����C
����֪ǿ��A��������̬���ʵ�ƽ��ʽ��(ƽ����Է�������)Ϊ26.3��
(1)�ƶ�A��B��C��D�Ļ�ѧʽ��A________��B________��C________��D________��
(2)д���٢ڱ仯�����ӷ���ʽ��
��____________________________________________________��
��_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��˳�ظ���ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
(ÿ��1��,��6��)�������б仯�����ƶϡ�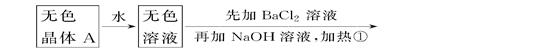 \x(����B����HClð���̰�ɫ����C
\x(����B����HClð���̰�ɫ����C
����֪ǿ��A��������̬���ʵ�ƽ��ʽ��(ƽ����Է�������)Ϊ26.3��
(1)�ƶ�A��B��C��D�Ļ�ѧʽ��A________��B________��C________��D________��
(2)д���٢ڱ仯�����ӷ���ʽ��
��____________________________________________________��
��_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ��ɽ��ѧ�߶�10���¿���ѧ�Ծ����������� ���ͣ������
(ÿ��1�� ��6��) һ���ѧ�о��ɹ�������ͭ��������(CuMn2O4)���ڳ����´����������е�һ����̼�ͼ�ȩ(HCHO)��
(1) ��һ�����ʵ���Ũ�ȵ�Cu(NO3)2��Mn(NO3)2��Һ�м���Na2CO3��Һ�����ó������������գ����Ƶ�CuMn2O4��
�� Mn2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ ��
�� NO3���Ŀռ乹��Ϊ (����������)��
(2) ��ͭ��������Ĵ��£�CO��������CO2��HCHO��������CO2��H2O��
�ٸ��ݵȵ���ԭ����CO���ӵĽṹʽΪ ��
�� H2O������Oԭ�ӹ�����ӻ�����Ϊ ��
�� 1molCO2�к��еĦҼ���ĿΪ ��
(3) ��CuSO4��Һ�м������NaOH��Һ������[Cu(OH)4]2���������ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
(ÿ��1��,��6��)�������б仯�����ƶϡ�
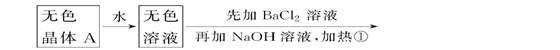 \x(����B����HClð���̰�ɫ����C
\x(����B����HClð���̰�ɫ����C
����֪ǿ��A��������̬���ʵ�ƽ��ʽ��(ƽ����Է�������)Ϊ26.3��
(1)�ƶ�A��B��C��D�Ļ�ѧʽ��A________��B________��C________��D________��
(2)д���٢ڱ仯�����ӷ���ʽ��
��____________________________________________________��
��_____________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com