
| ������ | Ag+��Mg2+��Al3+��Ba2+ | ||
| ������ | Cl-��
|


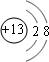
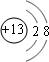
 �� ������������Al3+ ���ӽṹʾ��ͼΪ��
�� ������������Al3+ ���ӽṹʾ��ͼΪ��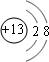 ��
�� ��
��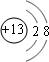 ��
��

 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� | �� | �� | �� |
�������и�Ԫ��ԭ�Ӹ����� | A��C | B��A | D��E | B��E |
1��1 | 1��2 | 1��3 | 1��4 |
����A��B����ԭ�ӵĺ˵����֮��������ǵ�ԭ������������֮�ͣ�Bԭ���������������������������2���������ڱ��У�C��E������Ԫ�أ���ѧ���ʻ��ã�D��E��λ��ͬһ���ڣ�D��E��ԭ������֮��Ϊ30��
��1��д��Ԫ�ط��ţ�A___________��B___________��C___________��D___________��E___________��
��2�����ˮ��Һ�м���MnO2����Ӧ�Ļ�ѧ����ʽ��____________________________��
��3����֪�ҵ�ʽ��С�ڼף���ʵ�����г���ʲô�����Ƶ��ң�______________________
��4������ˮ��Һ�����ԣ��뱥��NaHCO3��Һ��Ӧ��Ѹ�ٲ������������������й����ӷ���ʽΪ______________________��
��5����3 mL 1 mol��L-1KI��Һ������ע�������Լ�����1 mL 5 mol��L-1�����0.5 mL 30%����Һ����1 mL������������ã��ɵõ���ɫ��Һ��������ɫ��Һ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣�A��B��C��D��E���ֶ�����Ԫ�أ���֪�����ǵ�ԭ��������������A��B����Ԫ�صĺ˵����֮��������ǵ�ԭ������������֮�ͣ�Bԭ���������������������������2����Ԫ�����ڱ��У�C��E�IJ�ͬ��������Ԫ�أ�D��E��ԭ������֮��Ϊ30�����������γɵĻ�����Ϊ�ס��ҡ����������֡������ֻ�������ԭ�Ӹ��������±�������Ԫ�ط�������
|
| �� | �� | �� | �� |
| �������и�Ԫ��ԭ�Ӹ����� | A��C=1��1 | B��A=1��2 | D��E=1��3 | B��E=1��4 |
��1��д��A~E��Ԫ�ط���
A�� B�� C�� D�� E��
��2�����ˮ��Һ�м���MnO2������������ ��
��3����֪�л����ҵķ���Ϊƽ��ṹ��̼�������Ϊ120�㣬ʵ������ȡ�ҵĻ�ѧ����ʽΪ��
��4������ˮ��Һ�����ԣ��뱥��NaHCO3��Һ��Ӧ��������������������й����ӷ���ʽ�ǣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ��У������һ�δ�������ѧ�Ծ��������棩 ���ͣ������
�ס��ҡ���������������֮���������ת����ϵ��
��ش��������⣺
��1������Ϊ���Բ�������ת��Ϊ�ҵ����ӷ���ʽΪ ��
��2������Ϊ�γ��������Ҫ���ʣ���Ļ�ѧʽ ���������ȵ�NaOH��Һ��Ӧ��������Ԫ�ص����̬Ϊ+4��д���÷�Ӧ�����ӷ���ʽΪ��
��
��3�������к���Ŀǰ����ʹ����㷺�Ľ���Ԫ�أ�����ת���ɱ�Ϊ���Ϸ�Ӧ������Һ���������յõ��������� ����ȥ����Һ�������ҵķ����ǣ� ���û�ѧ����ʽ��ʾ������μ�������Һ�еı��������ʵ�鷽�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ������ʮУ�����������ѧ���ڳ�������ѧ�Ծ��������棩 ���ͣ��ƶ���
�ס��ҡ���������������֮���������ת����ϵ�� ��
��
��ش��������⣺
��1������Ϊ���Բ�������ת��Ϊ�ҵ����ӷ���ʽΪ ��
��2������Ϊ�γ��������Ҫ���ʣ���Ļ�ѧʽ ���������ȵ�NaOH��Һ��Ӧ��������Ԫ�ص����̬Ϊ+4��д���÷�Ӧ�����ӷ���ʽΪ ��
��3�������к���Ŀǰ����ʹ����㷺�Ľ���Ԫ�أ�����ת���ɱ�Ϊ���Ϸ�Ӧ������Һ���������յõ��������� ����ȥ����Һ�������ҵķ����� ���û�ѧ����ʽ��ʾ������μ�������Һ�еı��������ʵ�鷽�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com