
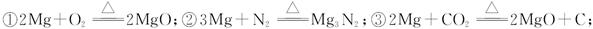

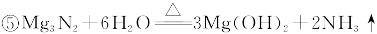
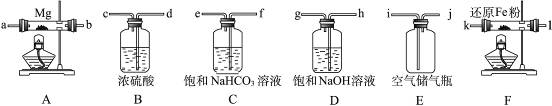



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
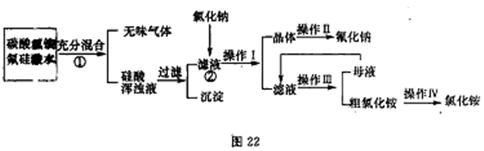
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
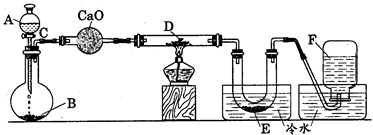 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ķ������������������� |
| B���ζ��ܡ�����ƿ��ʹ��ǰ�����ô�װҺ��ϴ |
| C����������NaOH��Һ����ˣ��Գ�ȥKCl��Һ��������MgCl2 |
| D���ⶨ����ͭ�����нᾧˮ���������������еľ��壬ʧˮ��Ӧ�ڿ�������ȴ�ٳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
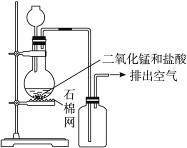
| A����װ��ͼ�����ٴ����������Դ��� |
| B����ʵ�����ռ������ķ�������ȷ |
| C��Ϊ�˷�ֹ������Ⱦ�������������β������ |
| D���ڼ���ƿ�ĵ��ܿڴ���һƬʪ��ĵ��۵⻯����ֽ����֤���Ƿ��������ݳ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
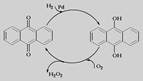
|

 (NH4)2S2O8 + H2����
(NH4)2S2O8 + H2����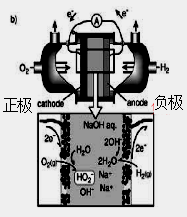
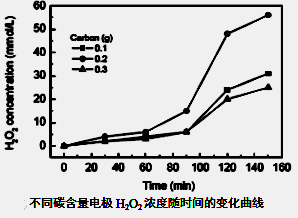
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ڹ���Ϊ��ʯ�� | B��ԭ������һ���� NO �� O2 |
| C��ԭ������һ����NH3��NO ��CO2 | D��ԭ�������� HC1�� Br2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
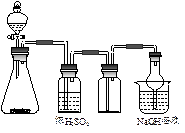
| A����ʯ��ˮ |
| B��MnO2��Ũ���� |
| C��CuƬ��Ũ���� |
| D��Na2SO3��Ũ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com