
ij������ȼ���ɼס��������л����϶��ɣ��ס����������ʺ���C��H��O����Ԫ���е����ֻ����֡���֪�ס��Ҽ�CO��H2��ȼ�������£�
| ���� | �� | �� | CO | H2 |
| ȼ����/(kJ��mol��1) | 1 366 | 5 518 | 283 | 286 |
 �żӾ���ϵ�д�
�żӾ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ѣ�CH3OCH3����һ����Ҫ�ľ�ϸ������Ʒ������Ϊ�Ƕ�ʮһ��������DZ����ȼ��[ ��֪��CH3OCH3(g)+3O2(g)��2CO2(g)+3H2O��1�� ��H����1455kJ/mol ]��ͬʱ��Ҳ������Ϊ�������������ȴ�������ҵ���Ʊ������ѵ���Ҫ���������������Σ�
�ټ״�Һ����Ũ���������»�״������ڴ�������ֱ����ˮ�ƶ����ѣ� 2CH3OH 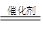 CH3OCH3��H2O
CH3OCH3��H2O
�ںϳ���CO��H2ֱ�Ӻϳɶ����ѣ� 3H2(g)��3CO(g) CH3OCH3(g)��CO2(g) ��H����247kJ/mol
CH3OCH3(g)��CO2(g) ��H����247kJ/mol
����Ȼ����ˮ������Ӧ�Ʊ������ѡ���CH4��H2OΪԭ���Ʊ������Ѻͼ״���ҵ�������£�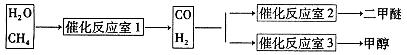
��1��д��CO(g)��H2(g)��O2(g)��Ӧ����CO2(g)��H2O��1�����Ȼ�ѧ����ʽ���������һλС����
��2���ڷ�Ӧ��2�У�һ�������·�����Ӧ3H2(g)��3CO(g) CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��
CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��
A�����¸�ѹ B���Ӵ��� C������COŨ�� D�������������
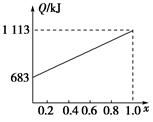
��3���ڷ�Ӧ��3�У���һ���¶Ⱥ�ѹǿ�����·����˷�Ӧ��3H2(g)��CO2(g)  CH3OH(g)��H2O (g) ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ���� ������ţ�
CH3OH(g)��H2O (g) ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ���� ������ţ�
A��P3��P2 T3��T2 B��P2��P4 T4��T2
C��P1��P3 T1��T3 D��P1��P4 T2��T3
��4����Ӧ��1�з�����Ӧ��CH4(g)��H2O(g) CO(g)��3H2(g) ��H��0д��ƽ�ⳣ���ı���ʽ��
CO(g)��3H2(g) ��H��0д��ƽ�ⳣ���ı���ʽ��
����¶Ƚ��ͣ��÷�Ӧ��ƽ�ⳣ�� ������䡱�����������С����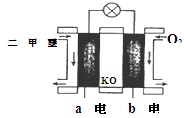
��5����ͼΪ��ɫ��Դ��������ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ��a�缫�ķ�ӦʽΪ��________________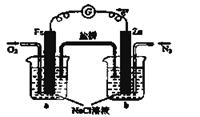
��6�������ж�����ȷ����_______
A�����ձ�a�м�������K3[Fe(CN)6]��Һ������ɫ��������
B���ձ�b�з�����ӦΪ2Zn-4e�� ��2Zn2+
C�����Ӵ�Zn������������Fe���������Żص�Zn��
D���ձ�a�з�����ӦO2 + 4H++ 4e�� �� 2H2O����ҺpH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���º���������,����Է�������ת��,�䷴Ӧ���̺�������ϵ��ͼ1��ʾ����֪:2SO2(g)+O2(g) 2SO3(g)��
2SO3(g)��
��H="-196.6" kJ/mol��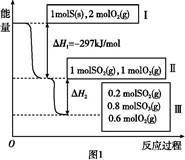

��ش���������:
(1)д���ܱ�ʾ���ȼ���ȵ��Ȼ�ѧ����ʽ: ��
(2)��H2=����������������������
(3)���º���ʱ,1 mol SO2��2 mol O2��ַ�Ӧ,�ų���������ֵ�ȨO��H2�O��������(�����С������ȡ�)��
(4)�����еĻ������ͨ��������NaOH��Һ������NaOH�����ʵ���Ϊ��������,����Һ�з�����������ԭ��Ӧ,��ù��̵����ӷ���ʽΪ�� ��
(5)����������,���д�ʩ����ʹn(SO3)/ n(SO2)�����������������
a.�����¶�
b.����He��
c.�ٳ���1 mol SO2(g)��1 mol O2(g)
d.ʹ�ô���
(6)ijSO2(g)��O2 (g)��ϵ,ʱ��t1�ﵽƽ���,�ı�ijһ�������,��Ӧ����v��ʱ��t�Ĺ�ϵ��ͼ2��ʾ,�����ı�SO2(g)��O2 (g)����,��ͼ��t4ʱ����ƽ���ƶ���������������������;ͼ�б�ʾƽ��������SO3�ĺ�����ߵ�һ��ʱ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Դ�Ŀ����������������Ŀɳ�����չϢϢ��ء�
��.��֪��Fe2O3��s����3C��s��=2Fe��s����3CO��g��
��H1��a kJ��mol��1
CO��g���� O2��g��=CO2��g���� ��H2��b kJ��mol��1
O2��g��=CO2��g���� ��H2��b kJ��mol��1
4Fe��s����3O2��g��=2Fe2O3��s���� ��H3��c kJ��mol��1
��C��ȼ���Ȧ�H��________kJ��mol��1��
��.��1������ԭ��صĹ���ԭ�������л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���________������ţ���
A��C��s����CO2��g��=2CO��g��
B��NaOH��aq����HCl��aq��=NaCl��aq����H2O��l��
C��2H2O��l��=2H2��g����O2��g��
D��2CO��g����O2��g��=2CO2��g��
�������ڵ�K2CO3��CO2Ϊ��Ӧ�Ļ�����������ѡ��Ӧ��Ƴ�һ��ԭ��أ���д����ԭ��صĸ�����Ӧ��_____________________________________��
��2��ijʵ��С��ģ�ҵ�ϳɰ���ӦN2��g����3H2��g�� 2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������
2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������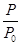 ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ش��������⣺
�ٷ�Ӧ��ƽ��ı�־��__________________________������ĸ���ţ���ͬ����
A��ѹǿ���ֲ���
B�������ܶȱ��ֲ���
C��NH3������������N2���������ʵ�2��
��0��2 min�ڣ���c��N2���仯��ʾ��ƽ����Ӧ����Ϊ________________��
�������N2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_____________________________��
A������ϵ�а������1��1�ٳ���N2��H2
B�������NH3
C�������¶�
D�����뺤��ʹѹǿ����
E������һ������N2
��3��25��ʱ��BaCO3��BaSO4���ܶȻ������ֱ���8��10��9��1��10��10��ij����BaCO3����������Һ�У�c��CO32-����0.2 mol��L��1���������������Na2SO4��Һ����Ҫ����BaSO4����������Na2SO4��Һ�����ʵ���Ũ����С��________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����֪�� C(s)+O2(g)=CO2(g) ��H1����393.5 kJ/mol
C(s)+H2O(g)=CO(g)+H2(g) ��H2����131.3 kJ/mol
��ӦCO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H= ____ ___kJ/mol��
��2����һ���ݵ��ܱ������У���CO��H2�ϳɼ״���CO(g)+2H2(g) CH3OH(g) ��H
CH3OH(g) ��H
���������β���˵���÷�Ӧ�Ѵﵽƽ��״̬����_______������ţ���
A��ÿ����1 mol CO��ͬʱ����2molH2
B��������������ʵ�������
C������CH3OH������������CO���������
D��CH3OH��CO��H2��Ũ�ȶ����ٷ����仯
��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��A��B�����ƽ�ⳣ��K(A)_______K(B)�����������=������,��ͬ������ͼ�жϦ�H _____0��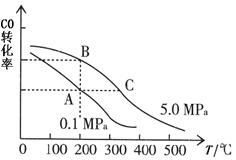
��ij�¶��£���2.0 mol CO��6.0 molH2����2 L���ܱ������У���ַ�Ӧ�ﵽƽ��ʱ���c(CO)="0.25" mol/L����CO��ת����= �����¶��µ�ƽ�ⳣ��K= ��������λ��Ч���֣���
��3�������¶�650���������ȼ�ϵ�أ���ú̿����CO��H2����������Ӧ�������CO2�Ļ������Ϊ������Ӧ����������缫����һ��������Li2CO3��Na2CO3���۵�����������ʡ������ĵ缫��ӦʽΪ��CO+H2��4e-+2CO32-=3CO2+H2O����õ�ص�������ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013������������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
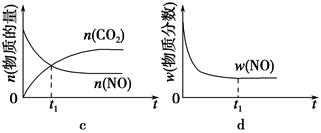
��1������β����������Ҫԭ��Ϊ2NO��g����2CO��g�� 2CO2��g����N2��g�������ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��
2CO2��g����N2��g�������ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯������ͼ��ʾ��
�ݴ��жϣ�
�ٸ÷�Ӧ�Ħ�H________0���>����<������
����T2�¶��£�0��2 s�ڵ�ƽ����Ӧ����v��N2����________��
�۵��������������һ��ʱ�����������������ѧ��Ӧ���ʡ��������ı����S1>S2������ͼ�л���c��CO2����T1��S2�����´ﵽƽ������еı仯���ߡ�
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����________������ţ���
��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
��úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ��
���磺CH4��g����2NO2��g��=N2��g����CO2��g����2H2O��g������H1����867 kJ��mol��1
2NO2��g�� N2O4��g������H2����56.9 kJ��mol��1
N2O4��g������H2����56.9 kJ��mol��1
д��CH4��g������ԭN2O4��g������N2��g����CO2��g����H2O��g�����Ȼ�ѧ����ʽ��__________________________________________________________________
�ڽ�ȼú�����Ķ�����̼�������ã��ɴﵽ��̼�ŷŵ�Ŀ�ġ���ͼ��ͨ���˹�������ã���CO2��H2OΪԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ������b���淢���ĵ缫��ӦʽΪ_______________________________________��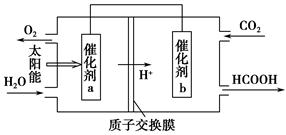
�۳����£�0.1 mol��L��1��HCOONa��ҺpHΪ10����HCOOH�ĵ��볣��Ka��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)(�㶫)����ʯ[��Ҫ�ɷ�Ca5(PO4)3F]�ڸ������Ʊ�����(P4)���Ȼ�ѧ����ʽΪ��4Ca5(PO4)3F(s)��21SiO2(s)��30C(s)===3P4(g)��20CaSiO3(s)��30CO(g)��SiF4(g)����H
��������Ӧ�У����������������________��
����֪��ͬ�����£�
4Ca5(PO4)3F(s)��3SiO2(s)===6Ca3(PO4)2(s)��2CaSiO3(s)��SiF4(g)����H1
2Ca3(PO4)2(s)��10C(s)===P4(g)��6CaO(s)��10CO(g)����H2
SiO2(s)��CaO(s)===CaSiO3(s)����H3
�æ�H1����H2�ͦ�H3��ʾ��H����H��____________��
(2)(����)��H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
��Cu(s)��2H��(aq)===Cu2��(aq)��H2(g) ��H1����64.39 kJ��mol��1
��2H2O2(l)===2H2O(l)��O2(g) ��H2����196.46 kJ��mol��1
��H2(g)�� O2(g)===H2O(l) ��H3����285.84 kJ��mol��1
O2(g)===H2O(l) ��H3����285.84 kJ��mol��1
��H2SO4��Һ�У�Cu��H2O2��Ӧ����Cu2����H2O���Ȼ�ѧ����ʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��Ӧ��Fe(s)+CO2(g) FeO(s)+CO(g)����H="a" kJ��mol-1,ƽ�ⳣ��ΪK;��Ӧ��CO(g)+1/2O2(g)
FeO(s)+CO(g)����H="a" kJ��mol-1,ƽ�ⳣ��ΪK;��Ӧ��CO(g)+1/2O2(g) CO2(g)����H="b" kJ��mol-1;��Ӧ��Fe2O3(s)+3CO(g)
CO2(g)����H="b" kJ��mol-1;��Ӧ��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H="c" kJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
2Fe(s)+3CO2(g)����H="c" kJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
| �¶�/�� | 500 | 700 | 900 |
| K | 1.00 | 1.47 | 2.40 |

 2FeO(s)�Ħ�H=����������
2FeO(s)�Ħ�H=���������� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����β����NOx�����������������������㷺��ע��
��1��ij��ȤС����������������Ϣ��
N2(g)+O2(g)=2NO(g) ��H=+180.5kJ/mol
2H2(g)+O2(g)=2H2O(g)) ��H=�D483.6kJ/mol
��Ӧ2H2(g)+2NO(g)=2H2O(g)+N2(g) ��H= ��
��2����С�����õ��ԭ���������ͼ1װ�ý���H2��ԭNO��ʵ��[�����ӵ����Ե�SCY�մ�(�ܴ���H+)Ϊ���ʣ������ٱ�Ĥ���缫]��
�ٵ缫AΪ �����缫��ӦʽΪ ��
��3����������ԭNOԭ�����£�
����Ӧ��4NO(g)+4NH3(g)+O2(g) 4N2(g)+6H2O(g) (��H <0)
4N2(g)+6H2O(g) (��H <0)
����Ӧ��4NH3(g)+3O2(g) 2N2(g)+6H2O(g)
2N2(g)+6H2O(g)
4NH3(g)+ 4O2(g) 2N2O(g)+6H2O(g)
2N2O(g)+6H2O(g)
4NO(g)+4NH3(g)+3O2(g) 4N2O(g)+6H2O(g)
4N2O(g)+6H2O(g)
�й�ʵ�������ͼ2��ͼ3��ʾ���ݴ˻ش��������⣺
�ٴ���ԭNOӦ����n(NH3)/n(NO)�����ֵΪ �������� ��
������Ӧƽ�ⳣ������ʽ��K= �������¶ȵ����ӣ�K�� (ѡ����ӡ��� ����С�����䡱��
��Ӱ��N2O�����ʵ������� ������Ũ�Ⱥ� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com