

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 O
O  Na2CO3
Na2CO3 ��CO2����H2O
��CO2����H2O  ��������Һ�����Ƚᾧ����
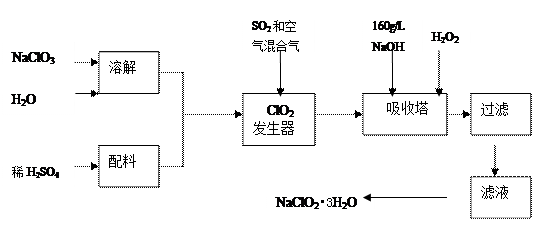
��������Һ�����Ƚᾧ���� ��ԭ��������̼�����Ƶ��Ʊ�ʵ�飬ͬѧ�ǰ�������Ƶķ���ʵ�顣
��ԭ��������̼�����Ƶ��Ʊ�ʵ�飬ͬѧ�ǰ�������Ƶķ���ʵ�顣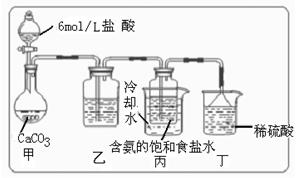
 ������װ���е��Լ���
������װ���е��Լ��� 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
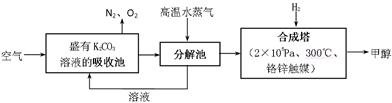
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
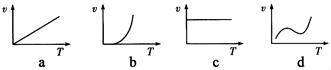
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 8SO2+2Fe2O3���÷�Ӧ������������ ��������8 mol SO2ʱת�Ƶ��ӵ����ʵ���Ϊ ��
8SO2+2Fe2O3���÷�Ӧ������������ ��������8 mol SO2ʱת�Ƶ��ӵ����ʵ���Ϊ �� 2SO3��ij�Ƽ�С���ͬѧ��һ�����º��ݵ�������ģ��÷�Ӧ�����Ƿ����ν���ʵ�飬��һ���������м���2 mol SO2��1 mol O2����Ӧ��ƽ�����SO2��ת����Ϊ��1���ڶ����������м���3 mol SO2��1.5 mol O2, ��Ӧ��ƽ�����SO2��ת����Ϊ��2�����1 ��2������ڡ��������ڡ���С�ڡ�����
2SO3��ij�Ƽ�С���ͬѧ��һ�����º��ݵ�������ģ��÷�Ӧ�����Ƿ����ν���ʵ�飬��һ���������м���2 mol SO2��1 mol O2����Ӧ��ƽ�����SO2��ת����Ϊ��1���ڶ����������м���3 mol SO2��1.5 mol O2, ��Ӧ��ƽ�����SO2��ת����Ϊ��2�����1 ��2������ڡ��������ڡ���С�ڡ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
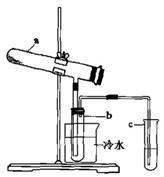
 Fe2O3+SO2+SO3��+14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թ�
Fe2O3+SO2+SO3��+14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թ� ��
��
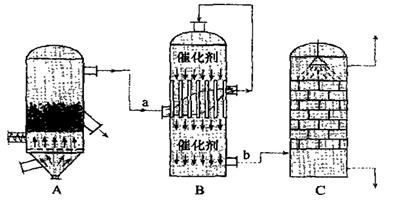
| A����������ĽӴ������ںϳ����з��� |
| B���������õ�������Ũ��Ϊ98�� |
| C�����պ���48���Ļ�����ʱ����FeS2��ʧ��2������S��ʧ4�� |
D�� Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת���� Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת���� |
 ���� ���� ���ɲ���������
���� ���� ���ɲ����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ܶ����γɹ��� | B�����ʵ�ȼ�� |
| C���ϳɸ߷��Ӳ��� | D����ʯ�ķ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com