
| �������� | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | Zn��OH��2 |
| ��ʼ������pH | 1.5 | 6.5 | 4.2 | 5.4 |
| ������ȫ��pH | 3.3 | 9.7 | 6.7 | 8.2 |
���� �������ʱSiO2���ܽ⣬���˷��룬����AΪSiO2����Һ�м������������г��̣���ͨ��������ҺpH��ʹFe3+ת��ΪFe��OH��3���������˷��룬��Һ���ټ���ZnS��Cu2+ת��ΪCuS���������˳�ȥ����Һ��ע��Ϊ�Ȼ�п���������淋õ�����п���壬���յ���Һ�к����Ȼ�淋ȣ�
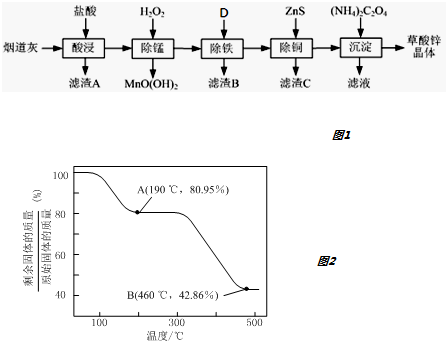
��1���ɷ�����֪������AΪ�������裬���Ӱ�컯ѧ��Ӧ���ʵ����ؽ��
��2�����̹����в���MnO��OH��2���������ݵ���غ�Ӧ�����������ɣ�
��3������ʹ�������������ʣ����ú���п����������̼���Σ����̲��ܵߵ���ZnS�ὫFe3+��ԭΪFe2+��ʹ��Ԫ�����Գ�ȥ��
��4����Na2C2O4���棨NH4��2C2O4��������п���壬���ɲ���識����Ȼ�п��Һ�����ɲ���п������
��5��ZnC2O4•2H2O�����е�ZnC2O4��������Ϊ$\frac{153}{153+36}$��100%=80.95%����A����ȫʧȥ�ᾧˮ����ѧʽΪZnC2O4������B��ΪZnO�����������ռ�е���������Ϊ$\frac{81}{153+36}$��100%=42.86%����B���������ΪZnO�����ԭ���غ��֪�����ɵ����ʵ�����CO��CO2��
��� �⣺��1���ɷ�����֪������AΪSiO2��Ϊ����߽������ʣ������̵��Ҵ����ø�ϸ�⣬���ɲ�ȡ�Ĵ�ʩ���ʵ����������Ũ�ȡ���߷�Ӧ�¶ȡ�����ȣ�
�ʴ�Ϊ��SiO2���ʵ����������Ũ�ȡ���߷�Ӧ�¶ȡ����裻
��2�����̹����в���MnO��OH��2���������ݵ���غ�Ӧ�����������ɣ���Ӧ���ӷ���ʽΪ��Mn2++H2O2+H2O=MnO��OH��2��+2H+��
�ʴ�Ϊ��Mn2++H2O2+H2O=MnO��OH��2��+2H+��
��3���ٳ���ʹ�������������ʣ�����ZnO��ZnCO3��Zn��OH��2�ȣ��ʴ�Ϊ��ZnO��ZnCO3��Zn��OH��2��
�ڳ������ͭ��˳���ܵߵ�����������ʻ��С����ԭ���ǣ��ȼ���ZnS�ὫFe3+��ԭΪFe2+��ʹ��Ԫ�����Գ�ȥ��
�ʴ�Ϊ���ȼ���ZnS�ὫFe3+��ԭΪFe2+��ʹ��Ԫ�����Գ�ȥ��
��4�����������̲���Na2C2O4���棨NH4��2C2O4��������п���壬�����ļ��Ϸ�ʽ�ǣ��ڽ����£���Na2C2O4�������뵽ZnCl2��Һ�У�
�ʴ�Ϊ����Na2C2O4�������뵽ZnCl2��Һ�У��ӱ߽��裻
��5��ZnC2O4•2H2O�����е�ZnC2O4��������Ϊ$\frac{153}{153+36}$��100%=80.95%����A����ȫʧȥ�ᾧˮ����ѧʽΪZnC2O4������B��ΪZnO�����������ռ�е���������Ϊ$\frac{81}{153+36}$��100%=42.86%����B���������ΪZnO�����ԭ���غ��֪�����ɵ����ʵ�����CO��CO2��300�桫460�淶Χ�ڣ�������Ӧ�Ļ�ѧ����ʽΪ��ZnC2O4$\frac{\underline{\;\;��\;\;}}{\;}$ZnO+CO��+CO2����
�ʴ�Ϊ��ZnC2O4$\frac{\underline{\;\;��\;\;}}{\;}$ZnO+CO��+CO2����
���� ���⿼�������Ʊ��������̣�Ϊ�߿��������ͣ�����ؼ��ǶԹ������̵����⣬�漰İ������ʽ��д�����ʵķ����ᴿ���������Ŀ������������ѧ����ȣ��Ƕ�ѧ���ۺ������Ŀ��飬�Ǹ߿�������Ŀ����������Ԫ�ػ��������ʣ���Ŀ�Ѷ��е�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | A2��g��+B2��g��?2AB��g����H��0 | B�� | 4NH3��g��+5O2��g��?4NO��g��+6H2O��g����H��0 | ||
| C�� | W��g��?Z��g����H��0 | D�� | 2SO3��g��?2SO2��g��+O2��g����H��0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ħ���������22.4 L/mol | |
| B�� | 1 mol H2������ֻ���ڱ���²�ԼΪ2 g | |
| C�� | ����£�18��H2O�����Ϊ22.4 L | |
| D�� | H2��O2�Ļ������1 mol�ڱ���µ����ԼΪ22.4 L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | AgCl������ˮ������ת��ΪAgI | |
| B�� | �ں���Ũ�Ⱦ�Ϊ0.001 mol•L-1��Cl-��I-����Һ�л�������AgNO3ϡ��Һ����������AgI���� | |
| C�� | AgI��AgCl��������ˮ�����ԣ�AgCl����ת��ΪAgI | |
| D�� | �����£�AgCl��Ҫ��NaI��Һ�п�ʼת��ΪAgI����NaI��Ũ�ȱ��벻����$\frac{1}{\sqrt{1.8}}$��10-11mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 +
+ $?_{��}^{ŨH_{2}SO_{4}}$
$?_{��}^{ŨH_{2}SO_{4}}$ +H2O
+H2O| ��Է������� | �ܶ�/��g•cm-3�� | �е�/�� | ˮ���ܽ��� | |
| ���촼 | 88 | 0.8123 | 131 | �� |
| ���� | 60 | 1.0492 | 118 | �� |
| ���������� | 130 | 0.8670 | 142 | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
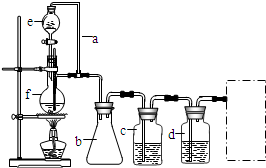 ijѧϰС���������кϳ�·�ߺϳ�1-������
ijѧϰС���������кϳ�·�ߺϳ�1-������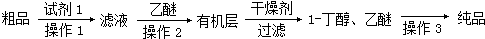
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.98 | 15.20 | 15.12 | 15.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH��7 | B�� | pH=7 | C�� | pH��7 | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͬ��ͬѹ�£���ͬ��������ʣ����ǵ����ʵ�������� | |
| B�� | 1 Lһ����̼����һ����1 L����������С | |
| C�� | 28 g N2��CO�Ļ�������ڱ�״���µ����ԼΪ22.4 L | |
| D�� | ���³�ѹ�£�1 mol̼��ȫȼ������22.4 L���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com