
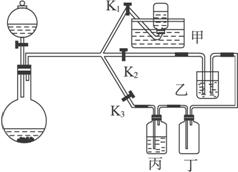
(1)��ͨ������____________װ�õ�Һ�壬���ɽ���SO2������ʵ�顣
��ͨ��Ʒ����Һ�е�������___________________________________________________��
(2)ʵ������У�ʹ�÷�Һ©���μ�Ũ����IJ���Ϊ______________________________��
(3)��Ҫ�ռ�����������SO2���壬Ӧ�Ƚ���SO2������ʵ�飬Ȼ��ر�___________����______________��װ�ü��е�Һ��Ϊ________________________��
(4)��Ҫ�ռ������SO2���壬װ�ñ���Ӧʢװ_______________��
(5)װ��������ʢҺ��Ϊ____________��Ŀ����__________________________________��
(6)ijһС����ʵ���з��֣�SO2����������������º���ʵ������ܲ����ԣ����ֲ��������������⣬�����Ʋ���ܵ�ԭ��˵����Ӧ����֤����(�ɲ�����)��
��ԭ��_______________����֤������___________________________________��
��ԭ��_______________����֤������___________________________________��
(1)�ٱ� ��Ʒ����Һ����ɫ�ɺ�ɫ��Ϊ��ɫ
(2)��Һ©���ϿڵIJ������ӣ�������Һ©���������������μ�
(3)K2��K3 K1 NaHSO3������Һ
(4)ŨH2SO4
(5)NaOHŨ��Һ ����SO2����
(6)��Na2SO3���ʣ�������ΪNa2SO4 ȡ�����������Թ��У�������������ˮ�ܽ⣬�ȵ����������ᣬ�ٵ���BaCl2��Һ���а�ɫ�������ɣ���֤��Na2SO3����
�ڲ���ŨH2SO4 �ýྻ������պȡ����Һ��Ϳ�ڰ�ֽ�ϲ���ڣ���֤����Һ����ŨH2SO4
������������һ��ʵ���ۺ��⣬���������ʵ��Ʊ������ʡ�β�����պ����ʵļ����֪ʶ���ؼ�ҪŪ���ΪSO2���ռ�װ�ã���ΪSO2������װ�ã���ΪSO2������ʵ��װ�ã���(6)С����ʵ���������ԣ����ֲ��������������⣬���ܵ�ԭ��ֻ�ܴ�ҩƷNa2SO3�Ƿ�����ΪNa2SO4����Һ�Dz���ŨH2SO4��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com