
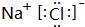 ��
������ W��X��Y��Z��ԭ���������������ͬһ����Ԫ�أ�W��X�ǽ���Ԫ�أ�Y��Z�Ƿǽ���Ԫ�أ��ɣ�4����W��X���Ե��������Ӧ��ˮ������Է�Ӧ�����κ�ˮ������֪WΪNa��XΪAl���ɣ�1����W��Z���γɻ�����WZ����Z����-1�ۣ���ZΪCl���ɣ�6����W��Na����Y���γɻ�����W2Y��Y����-2�ۣ���YΪS��
��� �⣺W��X��Y��Z��ԭ���������������ͬһ����Ԫ�أ�W��X�ǽ���Ԫ�أ�Y��Z�Ƿǽ���Ԫ�أ��ɣ�4����W��X���Ե��������Ӧ��ˮ������Է�Ӧ�����κ�ˮ������֪WΪNa��XΪAl���ɣ�1����W��Z���γɻ�����WZ����Z����-1�ۣ���ZΪCl���ɣ�6����W��Na����Y���γɻ�����W2Y��Y����-2�ۣ���YΪS��
��1��W��Z���γɻ�����WZΪNaCl���û�����ĵ���ʽΪ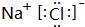 ��
��
�ʴ�Ϊ��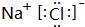 ��
��
��2��ͬһ����Ԫ���У�Ԫ�صķǽ���������ԭ���������������ǿ��Ԫ�صķǽ�����Խǿ�����⻯��Խ�ȶ��������ȶ���ΪHCl��H2S��
�ʴ�Ϊ��HCl��H2S��
��3���ǽ���Cl��S��������������ˮ��������ԣ�HClO4��H2SO4��
�ʴ�Ϊ��HClO4��H2SO4��
��4��W��X���Ե�����������Ӧ��ˮ����ֱ�ΪNaOH��Al��OH��3�����߷�Ӧ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��5��Z�����������Ϊ��Cl2O7��Cl2O7��ˮ��Ӧ���ɸ����ᣬ0.5molCl2O7��ˮ��Ӧ�ų�QkJ��������1molCl2O7��ˮ��Ӧ�ų�2QkJ�������������Ȼ�ѧ��Ӧ����ʽΪ��Cl2O7��l��+H2O��l��=2HClO4��aq����H=-2QkJ/mol��
�ʴ�Ϊ��Cl2O7��l��+H2O��l��=2HClO4��aq����H=-2QkJ/mol��
��6��0.1mol•L-1NaHSO4��0.2mol•L-1Na2S��Һ�������Ϻ�Ϊ��Ũ�ȵ�Na2SO4��NaHS��Na2S�����Һ����Һ��ʹpH��ֽ��������Һ�Լ��ԣ�HS-�ĵ���̶�С��S2-��ˮ��̶ȣ�
��ˮ��̶�������Һ������������Դ��ˮ�ĵ��롢��������HS-��ˮ�⣬��Һ������Ũ�ȴ�СΪ��
c��HS-����c��SO42-����c��S2-����c��OH-����c��H+�����ʴ���
�ڸ��������غ㣺2c��SO42-��=c��S2-��+c��HS-��+c��H2S������B��ȷ��
�۸��ݵ���غ㣺c��Na+��+c��H+��=2c��SO42-��+2c��S2-��+c��HS-��+c��OH-�����ʴ���
���������غ㣺3c��Na+��=5[c��SO42-��+c��S2-��+c��HS-��+c��H2S��]����������غ�ɵã�3c��H+��+2c��HS-��+5c��H2S��=c��S2-��+c��SO42-��+3c��OH-�����ʴ���
��ѡ���ڣ�
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��漰����ʽ������ˮ�⡢�Ȼ�ѧ����ʽ��д�ȣ��ؼ��Ǹ��������ƶ�Ԫ�أ���6��������Ũ�ȴ�С�Ƚ�Ϊ�״��㡢�ѵ㣬�ѶȽϴ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�2.24L�����к���ԭ����Ϊ0.4NA | |
| B�� | 0.3molNO2��������ˮ��Ӧת�Ƶ�����Ϊ0.2NA | |
| C�� | 1L0.1mol•L-1Na2CO3��Һ�к�CO32-��ĿΪ0.1NA | |
| D�� | 5.6g����ȫ��������ϡ���ᣬת�Ƶ�����Ϊ0.3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
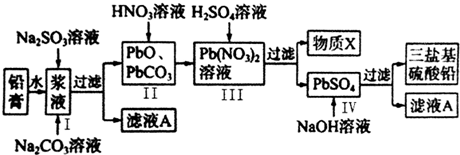
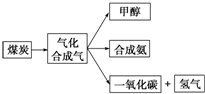 ��1����ͼ��ijú������ҵ����һ���֣�
��1����ͼ��ijú������ҵ����һ���֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��CH3��2 CH CH2CH2CH3B | B�� | ��CH3 CH2��2 CHCH3 | ||
| C�� | ��CH3��2 CH CH ��CH3��2 | D�� | ��CH3��3 C CH2CH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe3+ | B�� | Al3+ | C�� | Na+ | D�� | Fe2+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com