
”¾ĢāÄæ”æŅŃÖŖÓŠ»śĪļA”¢B”¢C”¢D”¢E”¢FÓŠŅŌĻĀ×Ŗ»Æ¹ŲĻµ”£AµÄ²śĮææÉŅŌŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½£»EŹĒ²»ČÜÓŚĖ®ĒŅ¾ßÓŠĻćĪ¶µÄĪŽÉ«ŅŗĢ壬Ļą¶Ō·Ö×ÓÖŹĮæŹĒCµÄ2±¶£»FĪŖøß·Ö×Ó»ÆŗĻĪļ”£½įŗĻĻĀĶ¼¹ŲĻµ»Ų“šĪŹĢā£ŗ
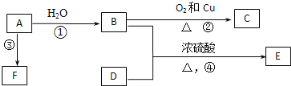
£Ø1£©Š“³öCµÄ½į¹¹¼ņŹ½£ŗ___________”£
£Ø2£©Š“³öB”¢DÖŠ¹ŁÄÜĶŵÄĆū³Ę£ŗB____________£¬D_____________”£
£Ø3£©Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
¢Ś_________________________”£
¢Ü________________________”£
£Ø4£©ĪļÖŹBæÉŅŌÖ±½Ó±»Ńõ»ÆĪŖD£¬Šč¼ÓČėµÄŹŌ¼ĮŹĒ____________________________”£
”¾“š°ø”æCH3CHOōĒ»łōČ»ł2CH3CH2OH+O2![]() 2CH3CHO+2H2OCH3COOH+CH3CH2OH
2CH3CHO+2H2OCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2OĖįŠŌøßĆĢĖį¼ŲČÜŅŗ£Ø»ņĖįŠŌÖŲøõĖį¼ŲČÜŅŗ£©
CH3COOCH2CH3+H2OĖįŠŌøßĆĢĖį¼ŲČÜŅŗ£Ø»ņĖįŠŌÖŲøõĖį¼ŲČÜŅŗ£©
”¾½āĪö”æ
AµÄ²śĮæĶس£ÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅµÄŹÆÓĶ»Æ¹¤Ė®Ę½µÄ±źÖ¾£¬ŌņAĪŖCH2=CH2£¬CH2=CH2ÓėĖ®·¢Éś¼Ó³É·“Ӧɜ³ÉBĪŖCH3CH2OH£¬CH3CH2OHŌŚCu×÷“߻ƼĮĢõ¼žĻĀ·¢Éś“ß»ÆŃõ»ÆÉś³ÉCĪŖCH3CHO£¬EŹĒ²»ČÜÓŚĖ®ĒŅ¾ßÓŠĻćĪ¶µÄĪŽÉ«ŅŗĢ壬Ļą¶Ō·Ö×ÓÖŹĮæŹĒCµÄ2±¶£¬ŌņEĪŖCH3COOC2H5£¬DĪŖCH3COOH£¬ŅŅĻ©·¢Éś¼Ó¾Ū·“Ӧɜ³ÉFĪŖ![]() £¬¾Ż“Ė½ā“š”£
£¬¾Ż“Ė½ā“š”£
øł¾ŻŅŌÉĻ·ÖĪöæÉÖŖAĪŖCH2=CH2£¬BĪŖCH3CH2OH£¬CĪŖCH3CHO£¬DĪŖCH3COOH£¬EĪŖCH3COOC2H5£¬FĪŖ![]() £¬Ōņ
£¬Ōņ
£Ø1£©CŹĒŅŅČ©£¬½į¹¹¼ņŹ½ĪŖCH3CHO£»
£Ø2£©BĪŖCH3CH2OH£¬ŗ¬ÓŠµÄ¹ŁÄÜĶÅĪŖōĒ»ł£¬DĪŖCH3COOH£¬ŗ¬ÓŠµÄ¹ŁÄÜĶÅĪŖōČ»ł£»
£Ø3£©·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ2CH3CH2OH+O2![]() 2CH3CHO+2H2O£»·“Ó¦¢ÜµÄ»Æѧ·½³ĢŹ½ĪŖCH3COOH+CH3CH2OH
2CH3CHO+2H2O£»·“Ó¦¢ÜµÄ»Æѧ·½³ĢŹ½ĪŖCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O£»
CH3COOCH2CH3+H2O£»
£Ø4£©ŅŅ“¼æÉŅŌÖ±½ÓŃõ»ÆĪŖŅŅĖį£¬ŠčŅŖ¼ÓČėĒæŃõ»Æ¼Į£¬Ņņ“Ė¼ÓČėµÄŹŌ¼ĮæÉŅŌŹĒĖįŠŌKMnO4ČÜŅŗ£Ø»ņĖįŠŌK2Cr2O4ČÜŅŗ£©”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”潫Ņ»¶ØĮæµÄNaHCO3ŗĶNa2O2µÄ»ģŗĻĪļÖĆÓŚŅ»ĆܱÕČŻĘ÷ÖŠ³ä·Ö¼ÓČČ£¬·“Ó¦ÖŠ×ŖŅʵĵē×ÓŹżĪŖ1mol£¬ĻĀĮŠĖµ·ØŅ»¶ØÕżČ·µÄŹĒ£Ø””””£©
A. »ģŗĻĪļÖŠNaHCO3ŗĶNa2O2ĪļÖŹµÄĮæŅ»¶ØĻąµČ
B. ČŻĘ÷ÖŠæĻ¶ØÓŠ0.5molO2
C. ·“Ó¦ŗó£¬ČŻĘ÷ÖŠµÄ¹ĢĢåÖ»ÓŠNa2CO3
D. ·“Ó¦ŗó£¬ČŻĘ÷ÖŠŅ»¶ØƻӊH2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĘųĢåµÄĢå»żÖ÷ŅŖÓÉŅŌĻĀŹ²Ć“ŅņĖŲ¾ö¶Ø£ŗ£Ø £©
¢ŁĘųĢåµÄ·Ö×Ó¶ąÉŁ ¢ŚĘųĢå·Ö×ӵēóŠ”
¢ŪĘųĢå·Ö×Ó¼äµÄĘ½¾ł¾ąĄė ¢ÜĘųĢå·Ö×ÓµÄĻą¶Ō·Ö×ÓÖŹĮ森
A.¢Ł¢Ś
B.¢Ł¢Ū
C.¢Ś¢Ū
D.¢Ś¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æN2O5ŹĒŅ»ÖÖŠĀŠĶĻõ»Æ¼Į£¬ĘäŠŌÖŹŗĶÖʱøŹÜµ½ČĖĆĒµÄ¹Ų×¢”£
£Ø1£©Ņ»¶ØĪĀ¶ČĻĀ£¬ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠN2O5æÉ·¢ÉśĻĀĮŠ·“Ó¦£ŗN2O5(g)![]() 4NO2(g)+O2(g)£»”÷H>0
4NO2(g)+O2(g)£»”÷H>0
¢ŁČē±ķĪŖ·“Ó¦ŌŚT1 ĪĀ¶ČĻĀµÄ²æ·ÖŹµŃ鏿¾Ż£ŗ
t/s | 0 | 500 | 1000 |
c(N2O5)/mol”¤L-1 | 5.00 | 3.52 | 2.48 |
Ōņ500sÄŚN2O5µÄ·Ö½āĖŁĀŹĪŖ____________________”£
¢ŚŌŚT 2ĪĀ¶ČĻĀ£¬·“Ó¦1000sŹ±²āµĆNO2µÄÅضČĪŖ4.98 mol”¤L-1£¬ŌņT2________T1( Ģī>”¢<»ņ=)”£
£Ø2£©ĻÖŅŌH2”¢O2”¢ČŪČŚNa2CO3×é³ÉµÄČ¼ĮĻµē³Ų²ÉÓƵē½ā·ØÖʱøN2O5£¬×°ÖĆČēĶ¼ĖłŹ¾£¬ĘäÖŠYĪŖCO2”£
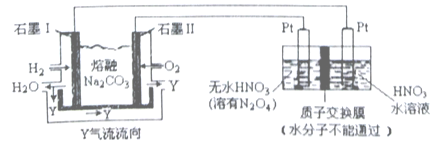
Š“³öŹÆÄ«¢ńµē¼«ÉĻ·¢Éś·“Ó¦µÄµē¼«·“Ó¦Ź½_____________________”£N2O5 ŌŚµē½ā³ŲµÄ_____________ĒųÉś³É(Ģī”°Ńō¼«”±»ņ”°Ņõ¼«”±)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻÖÓŠ½šŹōµ„ÖŹA”¢BŗĶĘųĢå¼×”¢ŅŅ”¢±ūŅŌ¼°ĪļÖŹC”¢D”¢E”¢F£¬ĖüĆĒÖ®¼äÄÜ·¢ÉśČēĻĀ·“Ó¦”££ØĶ¼ÖŠÓŠŠ©·“Ó¦µÄ²śĪļŗĶ·“Ó¦Ģõ¼žĆ»ÓŠ±ź³ö£©

Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AµÄ»ÆѧŹ½ĪŖ_____________£¬±ūµÄ»ÆѧŹ½ĪŖ_____________£»
£Ø2£©Š“³öĻĀĮŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
¢Ł _________________________________________£»
¢Ü _________________________________________£»
¢Ż _________________________________________”£
£Ø3£©Š“³öCČÜŅŗÓėAl·“Ó¦µÄ»Æѧ·½³ĢŹ½____________________”£
£Ø4£©ÅØĮņĖį¾ßÓŠĒæŃõ»ÆŠŌČ“æÉŅŌÓĆB²ÄĮĻ³µŌĖŹä£¬ŹĒŅņĪŖ_________________”£
£Ø5£©ĪŖ¼ų¶ØBµ„ÖŹ£¬½«ŹŌŃłÓĆĻ”ŃĪĖįČܽā£¬Č”ÉĻ²ćĒåŅŗŗóŠčŌŁ¼ÓČėµÄŹŌ¼Į£ØĢīŠ“×ÖÄø“śŗÅ£©ŹĒ_____________”£
A. µāĖ® B. NaOHČÜŅŗ C. KSCNČÜŅŗ D. Na2SO3ČÜŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¶ŌĖ®¹øµÄÖ÷ŅŖ³É·ÖŹĒCaCO3ŗĶMg(OH)2¶ų²»ŹĒCaCO3ŗĶMgCO3µÄ½āŹĶ£¬Ėµ·ØÕżČ·µÄŹĒ
A. Mg(OH)2µÄČܶȻż“óÓŚMgCO3µÄČܶȻż£¬ĒŅŌŚĖ®ÖŠ·¢ÉśĮĖ³Įµķ×Ŗ»Æ
B. Mg(OH)2±ČMgCO3øüÄŃČÜ£¬ĒŅŌŚĖ®ÖŠ·¢ÉśĮĖ³Įµķ×Ŗ»Æ
C. MgCO3µēĄė³öµÄCO![]() ·¢ÉśĖ®½ā£¬Ź¹Ė®ÖŠOH£ÅØ¶Č¼õŠ”£¬¶ŌMg(OH)2³ĮµķČܽāĘ½ŗā¶ųŃŌ£¬Qc£¼Ksp£¬Éś³ÉMg(OH)2³Įµķ
·¢ÉśĖ®½ā£¬Ź¹Ė®ÖŠOH£ÅØ¶Č¼õŠ”£¬¶ŌMg(OH)2³ĮµķČܽāĘ½ŗā¶ųŃŌ£¬Qc£¼Ksp£¬Éś³ÉMg(OH)2³Įµķ
D. ¶žÕß²»ÄÜĻą»„×Ŗ»Æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¹ŲÓŚCO2ÓėCS2µÄĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A. ĖüĆĒ»„ĪŖµČµē×ÓĢå B. CO2ĪŖÖ±ĻߊĪ¶ųCS2ĪŖVŠĪ
C. ĖüĆĒ·Ö×ÓÖŠµÄ»Æѧ¼üĄąŠĶ²»Ķ¬ D. CS2±ČCO2ĪȶØ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓŅĶ¼ŹĒŅ»øö»Æѧ¹ż³ĢµÄŹ¾ŅāĶ¼”£ŅŃÖŖ¼×³ŲµÄ×Ü·“Ó¦Ź½ĪŖ£ŗ2CH3OH+3O2+4KOH£½2K2CO3+6H2O”£
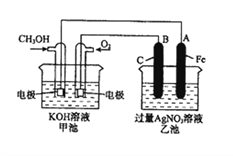
£Ø1£©Ēė»Ų“šĶ¼ÖŠ¼×”¢ŅŅĮ½³ŲµÄĆū³Ę”£¼×³ŲŹĒ________×°ÖĆ£¬ŅŅ³ŲŹĒ________×°ÖĆ”£
£Ø2£©Ēė»Ų“šĻĀĮŠµē¼«µÄĆū³Ę£ŗĶØČėCH3OHµÄµē¼«Ćū³ĘŹĒ__________________£¬B£ØŹÆÄ«£©µē¼«µÄĆū³ĘŹĒ______________________”£
£Ø3£©Š“³öµē¼«·“Ó¦Ź½£ŗĶØČėO2µÄµē¼«µÄµē¼«·“Ó¦Ź½ŹĒ__________________”£A£ØFe£©µē¼«µÄµē¼«·“Ó¦Ź½ĪŖ_______________________________________”£
£Ø4£©ŅŅ³ŲÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________________________________”£
£Ø5£©µ±ŅŅ³ŲÖŠA£ØFe£©¼«µÄÖŹĮæŌö¼Ó4.32gŹ±£¬¼×³ŲÖŠĄķĀŪÉĻĻūŗÄO2__________mL£Ø±ź×¼×“æöĻĀ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠŌŖĖŲÖŠ£¬×īøßÕż»ÆŗĻ¼ŪŹżÖµ×ī“óµÄŹĒ£Ø £©
A.NB.OC.FD.Al
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com