
(7��) Ϊ��ȥ�����е�Ca2+��Mg2+��SO42���Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������


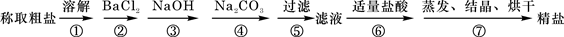
(1)�ж�BaCl2�ѹ����ķ�����_________________________________��
(2)�ڢܲ��У��йص����ӷ���ʽ��_____________________________��
(3)�����������pHֵ�ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����
_____________________________ ��
(4)Ϊ���龫�δ��ȣ�������1000mL.0.2 mol/L NaCl(����)��Һ��������ʱ�۲�Һ�����ӣ�����ͼ��ʾ��������������Ƶ���ҺŨ�ȣ���ƫ��ƫ�� ��________________��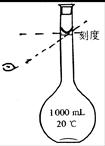
 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �¶ȡ� �ܽ�� ���� g/100gˮ |
10 | 20 | 30 | 40 | 50 | 60 | 70 |
| NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 37.8 |
| NH4HCO3 | 15.8 | 21.0 | 27.0 | ||||
| NaHCO3 | 8.2 | 9.6 | 11.1 | 12.7 | 14.4 | 16.4 | |
| NH4Cl | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.2 | 60.2 |
| c��V1 ��10-3��M(Na2CO3)g |
| Gg |
| c��V1 ��10-3��M(Na2CO3)g |
| Gg |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(7��) Ϊ��ȥ�����е�Ca2+��Mg2+��SO42���Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
(1)�ж�BaCl2�ѹ����ķ�����_________________________________��
(2)�ڢܲ��У��йص����ӷ���ʽ��_____________________________��
(3)�����������pHֵ�ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����
_____________________________ ��
(4)Ϊ���龫�δ��ȣ�������1000mL.0.2 mol/L NaCl(����)��Һ��������ʱ�۲�Һ�����ӣ�����ͼ��ʾ��������������Ƶ���ҺŨ�ȣ���ƫ��ƫ�� ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧ���������� ��ѧ��ɳ�����չ��ϰ���������棩 ���ͣ������
(8��)��ѡ����ʷ�������ĸ������գ�
(1)��ú��������ȡ���ױ�________��
(2)�Ӻ�ˮ����ȡ����________��
(3)ʮ����ת��Ϊ�������ϩ________��
(4)�����ת��Ϊ��ϩ����ϩ�Ȳ�������________��
(5)úת��Ϊ��̿��ú���͵�________��
(6)��ҵ������������ȡ������________��
(7)������ʯ����ȡ������________��
(8)�ᴿ������������(��ȥ���е���������)________��
A�����ˡ�B���ѽ⡡C������D���ѻ���E������F����ԭ��G����⡡H���ܽ⡡
I��������J��������K������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ��һ��ѧ�����п��Ի�ѧ�� ���ͣ������
(7��) Ϊ��ȥ�����е�Ca2+��Mg2+��SO42���Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
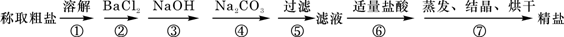
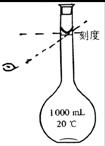
(1)�ж�BaCl2�ѹ����ķ�����_________________________________��
(2)�ڢܲ��У��йص����ӷ���ʽ��_____________________________��
(3)�����������pHֵ�ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����
_____________________________ ��
(4)Ϊ���龫�δ��ȣ�������1000mL.0.2 mol/L NaCl(����)��Һ��������ʱ�۲�Һ�����ӣ�����ͼ��ʾ��������������Ƶ���ҺŨ�ȣ���ƫ��ƫ�� ��________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com