

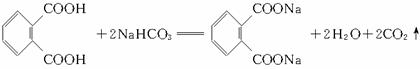
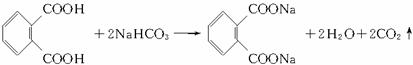
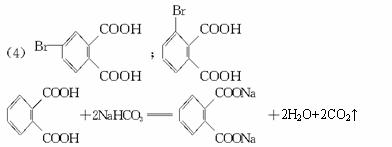
(1)�յ���һ�ŵĻ�ѧʽ(����ʽ)Ϊ______________________��
(2)���ʵ��������£�2-���Ӿ���Ӧ�ɵõ����㻯����E(C8H6O4)��1 mol E��������̼��������Һ��Ӧ�ɷų�������̼44.8 L(��״��)��E�������д�������ʱ��Ӧֻ����������һ��������һ�����Ľṹ��ʽ�ֱ���_____________________��________________________��E��̼�����Ʒ�Ӧ�Ļ�ѧ����ʽ��____________________________________��
(3)����E�������Ҵ���Ũ���������¼��ȣ���������һ����ѧʽ(����ʽ)ΪC12H14O4���»�����÷�Ӧ�Ļ�ѧ����ʽ��_______________________________����Ӧ����Ϊ___________��
(1)C16H12N2O

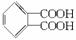
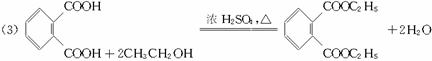 ������Ӧ
������Ӧ
������(1)��Ҫ�����Ԫ��ԭ�ӵijɼ����ɣ��յ���һ�ŵĻ�ѧʽֱ���ɽṹ��ʽ���C��H��N��O��ԭ�Ӹ�����(2)��Ҫ���������ƶ��л���Ľṹ��1 mol��E��������̼��������Һ��Ӧ�ɷų�2 mol������̼����֪E�к���������COOH������2�����Ӿ���Ӧ�ɵõ����㻯����E(C8H6O4)����E�Ľṹ��ʽΪ![]() ��1 mol��E�к���2 mol ��COOH����2 mol���Ҵ�����������Ӧ��������Ļ�ѧʽ����ΪC12H14O4��
��1 mol��E�к���2 mol ��COOH����2 mol���Ҵ�����������Ӧ��������Ļ�ѧʽ����ΪC12H14O4��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�յ���һ�ŵĻ�ѧʽ(����ʽ)Ϊ______________________________��
(2)���ʵ��������£�2-���Ӿ���Ӧ�ɵõ����㻯����E(C8H6O4)��1 mol E��������̼��������Һ��Ӧ�ɷų�������̼44.8 L(��״��)��E�������д�������ʱ��Ӧֻ����������һ��������һ�����Ľṹ��ʽ�ֱ���___________��___________��E��̼�����Ʒ�Ӧ�Ļ�ѧ����ʽ��______________________________��
(3)����E�������Ҵ���Ũ���������¼��ȣ���������һ����ѧʽ(����ʽ)ΪC12H14O4���»�����÷�Ӧ�Ļ�ѧ����ʽ��________________________________________ ����Ӧ������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
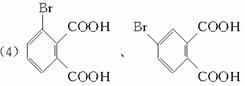
(1)�յ���һ�ŵĻ�ѧʽ(����ʽ)Ϊ____________��
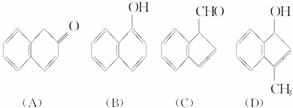
(2)�����滯����A��D�У���2-���ӻ�Ϊͬ���칹�����(����ĸ����) ____________ (��ʾ��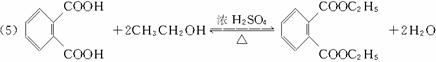 �ɱ�ʾΪ
�ɱ�ʾΪ![]() )
)
(3)����������(C)���еĹ�������____________________��
(4)���ʵ��������£�2-���Ӿ���Ӧ�ɵõ����㻯����E(C8H6O4)��1 mol E��������̼��������Һ��Ӧ�ɷų�������̼
(5)����E�������Ҵ���Ũ���������¼��ȣ���������һ����ѧʽ(����ʽ)ΪC12H14O4���»�����÷�Ӧ�Ļ�ѧ����ʽ��__________����Ӧ������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
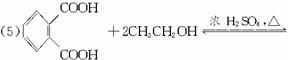
(1)�յ���һ�ŵĻ�ѧʽ(����ʽ)Ϊ___________��
(2)�����滯����(A)��(D)�У���2-���ӻ�Ϊͬ���칹�����(����ĸ����)___________��
(3)����������(C)���еĹ�������___________��
(4)���ʵ��������£�2-���Ӿ���Ӧ�ɵõ����㻯����E(C8H6O4)��1mol E��������̼��������Һ��Ӧ�ɷų�������̼
(5)����E�������Ҵ���Ũ���������¼��ȣ���������һ����ѧʽ(����ʽ)ΪC12H14O4���»�����÷�Ӧ�Ļ�ѧ����ʽ��______________________����Ӧ������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
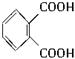
(1)�յ���һ�ŵĻ�ѧʽ(����ʽ)Ϊ_________��
(2)�����滯����(A)��(D)�У���2-���ӻ�Ϊͬ���칹�����(����ĸ����)______��(��ʾ�� �ɱ�ʾΪ
�ɱ�ʾΪ![]() )
)
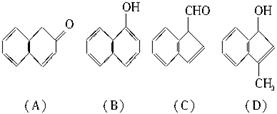
(3)����������(C)���еĹ�������______________��
(4)���ʵ��������£�2-���Ӿ���Ӧ�ɵõ����㻯����E(C8H6O4)��1 mol E��������̼��������Һ��Ӧ�ɷų�������̼44.8 L(��״��)��E�������д�������ʱ��Ӧֻ����������һ��ȡ�������һ��ȡ����Ľṹ��ʽ�ֱ���__________________��E��̼�����Ʒ�Ӧ�Ļ�ѧ����ʽ��___________________________��
(5)����E�������Ҵ���Ũ���������¼��ȣ���������һ����ѧʽ(����ʽ)ΪC12H14O4���»�����÷�Ӧ�Ļ�ѧ����ʽ��_______________________����Ӧ������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
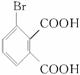

(1)�յ���һ�ŵĻ�ѧʽ(����ʽ)Ϊ______________��
(2)�����滯����A��D�У���2-���ӻ�Ϊͬ���칹�����(����ĸ����) _______��
(��ʾ��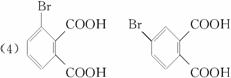
 �ɱ�ʾΪ
�ɱ�ʾΪ![]() )
)
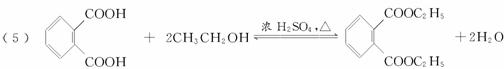
(3)����������C���еĹ�������___________��
(4)���ʵ��������£�2-���Ӿ���Ӧ�ɵõ����㻯����E(C8H6O4)��1 mol E��������̼��������Һ��Ӧ�ɷų�������̼44.8 L(��״��)��E�������д�������ʱ��Ӧֻ����������һ��ȡ�������һ��ȡ����Ľṹ��ʽ�ֱ���___________��E��̼�����Ʒ�Ӧ�Ļ�ѧ����ʽ�� ___________ ��
(5)����E�������Ҵ���Ũ���������¼��ȣ���������һ����ѧʽ(����ʽ)ΪC12H14O4���»�����÷�Ӧ�Ļ�ѧ����ʽ��_________________����Ӧ������_________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com