
��16�֣�Ϊ���������CO2�ĺ�����������⣬ij��ѧ���������ɫ���ɡ����룺�ѹ����ų��ĸ���CO2�ķ�������������̼�����Һ���գ�Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧʹ�����е�CO2ת��Ϊȼ�ϼ״�������ɫ���ɡ�����IJ��ּ����������£�
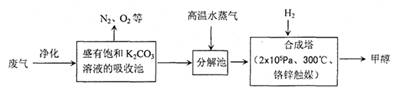
�ϳ����з�Ӧ�Ļ�ѧ����ʽΪ ����H<0����ƽ���ƶ�ԭ���������������������ԭ������ƽ��ת���ʡ���ʵ�������в���300����¶ȣ��������¶ȶԷ�Ӧ���ʵ�Ӱ���⣬����Ҫ������ ��
�Ӻϳ���������״���ԭ�������� ������ԭ���Ƚ����������ĸ����
A������ B����Һ C������ D���ᾧ
��3����ҵ������һ��������ѭ�����á�����ѭ�����á������Ч�桢���ܻ�������Ҫ��ʩ������ɫ���ɡ����뼼���������ܹ���ѭ�����á��ģ���K2CO3��Һ��CO2��H2�⣬������ ��
��4�������Ϊ2L�ĺϳ����У�����2 mol CO2��6 mol H2�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬v��H2) =______________����ʹƽ����ϵ��n(CH3OH)/n(CO2)����Ĵ�ʩ�� ��
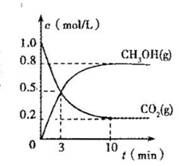
��5���罫CO2��H2��1:4������Ȼ�ϣ����ʵ��������¿��Ƶ�CH4��
��֪CH4(g)+2O2(g)= CO2(g)+2H2O(l) ��H1����890.3kJ/mol
H2 (g)+ O2(g)= H2O(l) ��H2����285.8kJ/mol
д��CO2(g)��H2(g)��Ӧ����CH4(g)��Һ̬ˮ���Ȼ�ѧ����ʽ________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| һ������ |
| һ������ |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ���ȼ�ѹ |
| ||
| ���ȼ�ѹ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
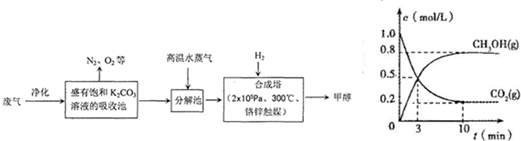
(14��) Ϊ���������CO2�ĺ�����������⣬ij��ѧ���������ɫ���ɡ����룺�ѹ����ų��ĸ���CO2�ķ�������������̼�����Һ���գ�Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧʹ�����е�CO2ת��Ϊȼ�ϼ״�������ɫ���ɡ�����IJ��ּ����������£�
��1���ϳ����з�Ӧ�Ļ�ѧ����ʽΪ ����H<0���÷�ӦΪ���淴Ӧ����ƽ���ƶ�ԭ���������������������ԭ������ƽ��ת���ʡ���ʵ�������в���300����¶ȣ��������¶ȶԷ�Ӧ���ʵ�Ӱ���⣬����Ҫ������ ��
��2���Ӻϳ���������״���ԭ�������� ������ԭ���Ƚ����������ĸ��
A������ B����Һ C������ D���ᾧ
��ҵ������һ��������ѭ�����á�����ѭ�����á������Ч�桢���ܻ�������Ҫ��ʩ������ɫ���ɡ����뼼���������ܹ���ѭ�����á��ģ���K2CO3��Һ��CO2��H2�⣬������ ��
��3��һ�������£������Ϊ1 L���ܱ������г���1 molCO2��3 mol H2�����CO2��CH3OH(g)��Ũ����ʱ��仯������ͼ��ʾ�����������У���ȷ���� ��
A�������¶���ʹ����
B����Ӧ�ﵽƽ��״̬ʱ��CO2��ƽ��ת����Ϊ75%
C��3 minʱ����CO2��Ũ�ȱ�ʾ������Ӧ���ʵ�����CH3OH��Ũ�ȱ�ʾ���淴Ӧ����
D���ӷ�Ӧ��ʼ��ƽ�⣬H2��ƽ����Ӧ������(H2)��0.075 mol•L��1•min��1
(4) �罫CO2��H2��1:4������Ȼ�ϣ����ʵ��������¿��Ƶ�CH4��
��֪
д��C02(g)��H2(g)��Ӧ����CH4(g)��Һ̬ˮ���Ȼ�ѧ����ʽ
___________ _____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�����и�����ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�������
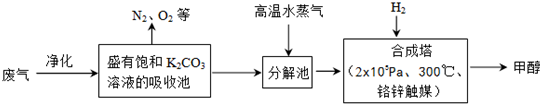
��12�֣�Ϊ���������CO2�ĺ�����������⣬ij��ѧ���������ɫ���ɡ����룺�ѹ����ų��ĸ���CO2�ķ�������������̼�����Һ���գ�Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧʹ�����е�CO2ת��Ϊȼ�ϼ״�������ɫ���ɡ�����IJ��ּ�����������
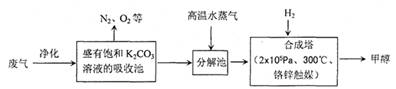
��1���ϳ����з�Ӧ�Ļ�ѧ����ʽΪ ����H<0����ƽ���ƶ�ԭ���������������������ԭ������ƽ��ת���ʡ���ʵ�������в���300����¶ȣ��������¶ȶԷ�Ӧ���ʵ�Ӱ���⣬����Ҫ������ ��
��2���Ӻϳ���������״���ԭ�������� ������ԭ���Ƚ����������ĸ��
A������ B����Һ C������ D���ᾧ
��ҵ������һ��������ѭ�����á�����ѭ�����á������Ч�桢���ܻ�������Ҫ��ʩ������ɫ���ɡ����뼼���������ܹ���ѭ�����á��ģ���K2CO3��Һ��CO2��H2�⣬������ .
��3�������Ϊ2L�ĺϳ����У�����2 mol CO2��6 mol H2�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��

�ӷ�Ӧ��ʼ��ƽ�⣬V��H2) =______________����ʹƽ����ϵ��nCH3OH)/n(CO2)����Ĵ�ʩ��______ __��
(4) �罫CO2��H2��1:4������Ȼ�ϣ����ʵ��������¿��Ƶ�CH4��
��֪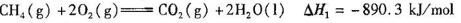

д��C02(g)��H2(g)��Ӧ����CH4(g)��Һ̬ˮ���Ȼ�ѧ����ʽ________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com