
���� ��1������CuCO3���ȷֽ�õ�CuO��CO2��Cu��OH��2���ȷֽ�õ�CuO��H2O��Ȼ��������CuO��Ӧ����ˮ������xCuCO3•yCu��OH��2•zH2O��������Ӧ�IJ����У�Cu��CO2��H2O�������ݽ���ʽ̼��ͭǰ��ϵ����Ϊ1�����ݷ�Ӧǰ���ԭ���������������ƽ��
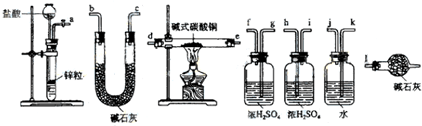
��2����ʵ���ԭ�����ø�����������ʽ̼��ͭ��Ӧ��ͨ���ⶨ���ɵĶ�����̼��ˮ�Լ���Ӧ������������ȷ�����ʵ���ɣ�Ϊ��ֹ������CO2��H2O����U����װ�ã��������������һ��ʢ�м�ʯ�ҵĸ���װ�������տ����еĶ�����̼��ˮ��
��3�����ݻ�ѧ����ʽ������֮���������ϵ���x��y��z���������ɵ�ˮ�ͷ�Ӧ����ˮ�Ĺ�ϵ���ˮ��������
��4�����ݷ�ӦxCuCO3•yCu��OH��2•zH2O=��x+y��CuO+xCO2��+��y+z��H2O�������ݼ�ʽ̼��ͭ��CuO��CO2��H2O�����������������������������ɼ��������ɣ�
��� �⣺��1��xCuCO3•yCu��OH��2•zH2O��������Ӧ�IJ����У�Cu��CO2��H2O������ʽΪ��xCuCO3•yCu��OH��2•zH2O+��x+y��H2=��x+y��Cu+xCO2+��x+2y+z��H2O���ʴ�Ϊ��xCuCO3•yCu��OH��2•zH2O+��x+y��H2=��x+y��Cu+xCO2+��x+2y+z��H2O��
��2����ʵ���ԭ�����ø�����������ʽ̼��ͭ��Ӧ��ͨ���ⶨ���ɵĶ�����̼��ˮ�Լ���Ӧ������������ȷ�����ʵ���ɣ�Ϊ��ֹ������CO2��H2O����U����װ�ã��������������һ��ʢ�м�ʯ�ҵĸ���װ�������տ����еĶ�����̼��ˮ�����������������˳����a��k��j��gf��hi����de��ed����hi��gf����bc��cb����l���ʴ�Ϊ��a��k��j��gf��hi����de��ed����hi��gf����bc��cb����l��
��3��xCuCO3•yCu��OH��2•zH2O+�� x+y��H2=��x+y��Cu+xCO2��+��x+2y+z��H2O
64��x+y�� 44x 18��x+2y+z��
9.6g 4.4g 5.4g
$\frac{64��x+y��}{9.6g}$=$\frac{44x}{4.4g}$����ã�2y=x
$\frac{44x}{4.4g}$=$\frac{18��x+2y+z��}{5.4g}$����ã�z=x
��x=2����y=1��z=2����ѧʽΪ2CuCO3•Cu��OH��2•2H2O����ѧ����ʽΪ2CuCO3•Cu��OH��2•2H2O+3H2=3Cu+2CO2��+6H2O���ɻ�ѧ����ʽ���Կ�������ʽ̼��ͭÿ2��ˮ����������6��ˮ���ӣ������ɵ�ˮ�м�ʽ̼��ͭ��ˮռ��$\frac{1}{3}$����˼�ʽ̼��ͭ��ˮ������Ϊ��5.4g��$\frac{1}{3}$=1.8g��
�ʴ�Ϊ��1.8��2CuCO3•Cu��OH��2•2H2O��
��4�����ݷ�ӦxCuCO3•yCu��OH��2•zH2O=��x+y��CuO+xCO2��+��y+z��H2O�������ݼ�ʽ̼��ͭ��CuO��CO2��H2O�����������������������������ɼ��������ɣ�
�ʴ�Ϊ�����У����ݷ�ӦxCuCO3•yCu��OH��2•zH2O=��x+y��CuO+xCO2��+��y+z��H2O�������ݼ�ʽ̼��ͭ��CuO��CO2��H2O�����������������������������ɼ��������ɣ�
���� ���⿼����������ɵ�ʵ��̽�����ؼ�����ƽ��ѧ����ʽ��������ʵ��ԭ��������㡢���ݻ�ѧ����ʽ���м��㣬ע�������Ϣ������Ӧ�ã���Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol�����������NA��������ӵ�������� | |
| B�� | NA���������Ӻ�NA���������ӵ������ȵ���16��1 | |
| C�� | 30g���飨C2H6��������ԭ����ĿΪNA | |
| D�� | �ڱ�״���£�0.5NA������������ռ���Լ��11.2L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ά����A����ͼ����һ�н�����Ƥ��֯��������ʣ�ȱ��ά����Aʱ�������ͯ��������������ҹä֢��Ƥ������ȶ��ֲ�֢��
ά����A����ͼ����һ�н�����Ƥ��֯��������ʣ�ȱ��ά����Aʱ�������ͯ��������������ҹä֢��Ƥ������ȶ��ֲ�֢���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ����һ��Ũ�ȵ�NaCl��Һ | B�� |  ʵ������ȡ�������� | ||
| C�� | 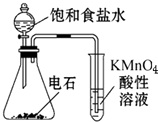 ������Ȳ�Ļ�ԭ�� | D�� | 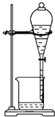 ����ˮ�����ᱽ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ��� | B�� | O2��O3 | C�� |  �� �� 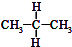 | D�� | 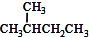 �� ��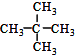 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ʒ�� | ������ |
| ���� | �ʼ���������ۡ���ɰ�ǡ�����ֲ���͡����͡��̷ۡ�ʳ�Ρ�����֭�� |
| ������ | 240�� |
| �������� | ���ڰ�װ������� |
| ֯�� | �� | �� | �� |
| ����ʱ ����ζ | �ս���ë ����ζ | ��ֽ����ζ | �������ζ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com