
���� ������480mL��Һ����Ҫѡ��500mL����ƿ������n=cV�����̼���Ƶ����ʵ������ٸ���m=nM�����̼���Ƶ�������
��ʵ����û��480mL����ƿ������ʱ��Ҫѡ�ù��Ϊ500mL������ƿ��
�����ƹ����б�������ձ��Ͳ�����2-3�Σ�����ϴ��Һת�Ƶ�����ƿ�У�
���������Ҫʹ�ý�ͷ�ιܣ�����ˮ�����̶��ߣ��˴�����ʧ�ܣ���Ҫ�������ã�
��ҡ�Ⱥ�����������ƿ�̶����Ϸ��в�����Һ������Һ�����ڿ̶��ߣ���������������Ҫ��������ˮ��
�ޣ�1�����ڶ���ʱ����Ҫ��������ˮ������ƿ�е���������ˮ��Ӱ�����ʵ����ʵ�����������Һ�����
��2���ȵ���Һ���ƫ����ȡ����Һ�����С���������Ƶ���Һ���ƫС��Ũ��ƫ��
��3����δ��ϴ�ӻ�ϴ��Һ��ת��ʱ����������ʵ�����ϣ��ᵼ�����Ƶ���Һ�����ʵ����ʵ���ƫС��
��4������ʱ���ӿ̶��ߣ��ᵼ�¼��������ˮ���ƫС�����Ƶ���Һ���ƫС����Һ���ƫ��
��� �⣺��ʵ����Ҫ����0.100mol/L��Na2CO3��Һ480mL����Ҫʹ��500mL��������ƿ���ƣ�500mL 0.100mol/L��Na2CO3��Һ�к���̼���Ƶ����ʵ���Ϊ��0.100mol/L��0.5L=0.05mol����Ҫ̼���Ƶ�����Ϊ��106g/mol��0.05mol=5.3g��
�ʴ�Ϊ��5.3��
�ڽ��ܽ⡢��ȴ�����Һת�Ƶ�500mL����ƿ�У�
�ʴ�Ϊ��500mL����ƿ��
�ܼ���������ƿ�л�����������ˮ��Һ�����̶���1��2cm�������ý�ͷ�ιܽ��ж��ݣ�Ҫ��εμ�����ˮ����Һ�İ�Һ��������̶������У����������ˮ����������ƿ�̶��ߣ��˴ε�����ʧ�ܣ���Ҫ�������������ƣ�
�ʴ�Ϊ���������ƣ�
�ݰ�����ƿ���Ǻã��������µߵ������ҡ�ȣ����������Һ����ڿ̶��ߣ�����������������������ڿ̶������ϵ���Һ�������䣬������̶�����ƽ����Ӱ����Һ�����������Ҫ��������ˮ�������ټ�ˮ���̶��ߵ���Ũ��ƫС��
�ʴ�Ϊ������Ҫ��
�ޣ�1������ƿ��ʹ��֮ǰ�����������ˮ���С���©������������������ƿ��ʪ������������ˮ����Ҫ���ʵ����ʵ�����������Һ��������Բ�Ӱ�����ƽ����
�ʴ�Ϊ��C��
��2����Щ�����ܽ����ȣ���δ����ȴ��ת��������ƿ���������Ƶ���Һƽ��ƫС��������Һ��Ũ�Ȼ�ƫ��
�ʴ�Ϊ��B��
��3����δ��ϴ�ӻ�ϴ��Һ��ת��ʱ����������ʵ�����ϣ��ᵼ�����Ƶ���Һ�����ʵ����ʵ���ƫС����������Һ��Ũ�Ȼ�ƫС��
�ʴ�Ϊ��A��
��4�����ڶ��ݵĹ���֮�У�����ʼ�ո��ӿ̶��ߣ����¼��������ˮ���ƫС����������Һ��Ũ�Ȼ�ƫ��
�ʴ�Ϊ��B��
���� ������һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ��ע��c=$\frac{n}{V}$����Ӧ�ã���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ��һ�ϵ�һ���¿���ѧ���������棩 ���ͣ�ʵ����
������ƿ ��������ƿ �������� ����Ͳ
���ձ� ��������ƽ �߷�Һ©�� ���Թ�
��1�������ڼ��ȵ����ص�ʯ�������� ��
��2��ʹ��ʱ�������Ƿ�©ˮ���� ��
��3��������̶ȵ��� ��
II����ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ����ѧʵ���ڻ�ѧѧϰ�о�����Ҫ���á����й���ʵ�����������ȷ����___________��
A����������ȼ�ŵľƾ���ʹ�ƾ���������ȼ��������Ӧ������ʪ�{����� |
B����������մ��Ƥ���������ϣ�Ӧ������ŨNaOH��Һ��ϴ |
C��ҹ���������ú��й©ʱ���������Ƽ��ú��й©ԭ���������Ŵ�ͨ�� |
D����������ԭ����ͭʱ���ȼ�������ͭ����ͨ������ |
E����ȼ�������ȼǰһ��Ҫ�鴿
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
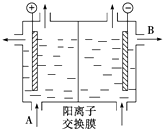 ��Ҫ��������и�С��
��Ҫ��������и�С���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | HClO2 | HF | HCN | H2S |
| Ka | 1��10-2 | 6.3��10-4 | 4.9��10-10 | K1=9.1��10-8K2=1.1��10-12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��1��ʵ�鲽�� | �й����� |
| �ټ�������Na2SO4������ | ��Ҫ����Na2SO4������Ϊ2.8g |
| �ڳ���Na2SO4���� | ������Ҫ�õ�����Ҫ�����ǣ�������ƽ |
| �۽�Na2SO4����100mL�ձ��У�����������ˮ | �ò�������������ȫ�ܽ⣬��ȴ������ |
| �ܽ��ձ�����Һת��������A�У��Ѽ�鲻©ˮ�� | ����A��100mL����ƿ�� |
| ��ϴ���ձ���ת�ƣ����� | |
| ��ҡ�ȡ�װƿ�����ϱ�ǩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ڢܢ� | D�� | �٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
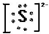
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com