
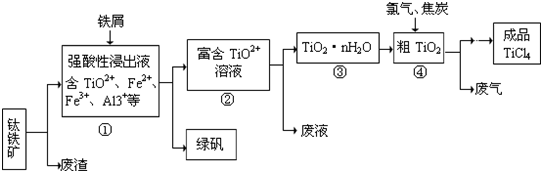
(9��) ���Ȼ�������ɫҺ�壬�е�Ϊ136�档������ˮ�⣬��������ˮ���������������̡���TiCl4+H2O=TiOCl2+2HCl��������650��~850���£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�һ���ж����塣��ͼ��ʵ�����Ʊ�TiCl4�ķ�Ӧװ�ã�����Ҫ�����������£�
�����Ӻ�����װ�ã���ͨCl2ǰ��ͨ��CO2���岢����һ��ʱ�䣻
�ڵ���ƿ��TiCl4������������ʱ��ֹͣ���ȣ��Ӳ���и�ͨCO2����ֱ����¯�еĴɹ���ȴΪֹ��
�۽�TiO2��̿�ۻ�Ͼ��Ⱥ�װ���ʽ��¯�У�
�� ����¯���µ�800�棬һ��ʱ����ͨCl2��ͬʱ����������ͨ����ˮ��
�Իش��������⣺
��1����ȷ�IJ���˳��Ϊ������ţ� ��
��2��װ��A�еķ�Ӧ�����ӷ���ʽΪ ��
��3��Cװ���еķ�Ӧ����ʽΪ�� ��
��4�������ٵ�Ŀ���� ��
��5��װ��D�������ܽ�ˮ�ڵ�λ���ǣ���a��b�� ��װ��E�������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| TiCl4 | SiCl4 | |
| �۵�/�� | -25.0 | -68.8 |
| �е�/�� | 136.4 | 57.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�Ƹ���ѧ����ʮ���¿���ѧ���Ծ� ���ͣ�ʵ����
(9��) ���Ȼ�������ɫҺ�壬�е�Ϊ136�档������ˮ�⣬��������ˮ���������������̡���TiCl4+H2O=TiOCl2+2HCl��������650��~850���£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�һ���ж����塣��ͼ��ʵ�����Ʊ�TiCl4�ķ�Ӧװ�ã�����Ҫ�����������£�
�����Ӻ�����װ�ã���ͨCl2ǰ��ͨ��CO2���岢����һ��ʱ�䣻
�ڵ���ƿ��TiCl4������������ʱ��ֹͣ���ȣ��Ӳ���и�ͨCO2����ֱ����¯�еĴɹ���ȴΪֹ��
�۽�TiO2��̿�ۻ�Ͼ��Ⱥ�װ���ʽ��¯�У�
�� ����¯���µ�800�棬һ��ʱ����ͨCl2��ͬʱ����������ͨ����ˮ��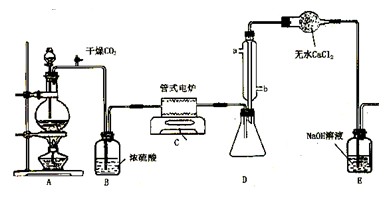
�Իش��������⣺
��1����ȷ�IJ���˳��Ϊ������ţ� ��
��2��װ��A�еķ�Ӧ�����ӷ���ʽΪ ��
��3��Cװ���еķ�Ӧ����ʽΪ�� ��
��4�������ٵ�Ŀ���� ��
��5��װ��D�������ܽ�ˮ�ڵ�λ���ǣ���a��b�� ��װ��E�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ����ʮ���¿���ѧ���Ծ� ���ͣ�ʵ����
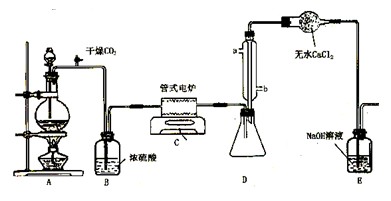
(9��) ���Ȼ�������ɫҺ�壬�е�Ϊ136�档������ˮ�⣬��������ˮ���������������̡���TiCl4+H2O=TiOCl2+2HCl��������650��~850���£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�һ���ж����塣��ͼ��ʵ�����Ʊ�TiCl4�ķ�Ӧװ�ã�����Ҫ�����������£�
�����Ӻ�����װ�ã���ͨCl2ǰ��ͨ��CO2���岢����һ��ʱ�䣻
�ڵ���ƿ��TiCl4������������ʱ��ֹͣ���ȣ��Ӳ���и�ͨCO2����ֱ����¯�еĴɹ���ȴΪֹ��
�۽�TiO2��̿�ۻ�Ͼ��Ⱥ�װ���ʽ��¯�У�
�� ����¯���µ�800�棬һ��ʱ����ͨCl2��ͬʱ����������ͨ����ˮ��
�Իش��������⣺
��1����ȷ�IJ���˳��Ϊ������ţ� ��
��2��װ��A�еķ�Ӧ�����ӷ���ʽΪ ��
��3��Cװ���еķ�Ӧ����ʽΪ�� ��
��4�������ٵ�Ŀ���� ��
��5��װ��D�������ܽ�ˮ�ڵ�λ���ǣ���a��b�� ��װ��E�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
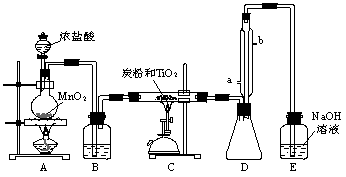 (�����9��)���Ȼ�������ɫҺ�壬�е�Ϊ136�棬������ˮ�⣬�������е�ˮ���������������̡�(TiCl4��H2O��TiOCl2��2HCl��)��TiCl4����TiO2��Cl2��Ӧ�Ƶ�(TiO2����HCl��Ӧ)���˷�Ӧ��1000������½��еĺ������������̿�۴���ʱ650�桫850�淴Ӧ���˳�����С���ͼ��ijͬѧ��Ƶ�ʵ�����Ʊ�TiCl4��װ�á���ش�
(�����9��)���Ȼ�������ɫҺ�壬�е�Ϊ136�棬������ˮ�⣬�������е�ˮ���������������̡�(TiCl4��H2O��TiOCl2��2HCl��)��TiCl4����TiO2��Cl2��Ӧ�Ƶ�(TiO2����HCl��Ӧ)���˷�Ӧ��1000������½��еĺ������������̿�۴���ʱ650�桫850�淴Ӧ���˳�����С���ͼ��ijͬѧ��Ƶ�ʵ�����Ʊ�TiCl4��װ�á���ش�
(1)Aװ���з�Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
(2)Bװ���е��Լ�Ϊ________________��������______________________________��
(3)ʵ����̿�۵�����Ϊ_______________________��
(4)Dװ��������ˮ��ˮ������Ϊ____________��____________������װ�õ�������_________________��
(5)Eװ����NaOH��Һ��������_______________________________________��
(6)����Ʒ����г���Һ©���е�Ũ�����˳�����¡�E���ܷ�ںͿ��ܵ����⣬����һ�����ԵIJ�����_______________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com