
 _____________��
_____________��
| �Լ������� | �����Լ�/g | NH3���/mL |
| a | 12.0g Ca(OH)2(����) 10.8g NH4Cl | 2688 |
| b | 12.0g Ca (OH)2(����) 10.8g(NH4)2SO4 (OH)2(����) 10.8g(NH4)2SO4 | 2728 |
| c | 12.0g NaOH(����) 10.8g NH4Cl | 3136 |
| d | 12.0g NaOH(����) 10.8g (NH4)2SO4 | 3118 |
| e | 12.0g CaO(����) 10.8g NH4Cl | 3506 |
| f | 12.0g CaO(����) 10.8g (NH4)2SO4 | 3584 |
 ____________��
____________��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��0�桫50�� | B��0�桫100�� | C��0�桫200�� | D��0�桫360�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ü�������ͭ�۵ķ�����ȥCu��NO3��2��Һ�л��е�AgNO3 |
| B����ϴ��ƿ�е�NaOH��Һ��ȥCO2�л��е�HCl���� |
C������NaOH��Һ�����ȣ�������������ʹʪ��ĺ�ɫʯ����ֽ������ȷ����Һ���� |
| D���Ⱥ�����ʯ����Һ��BaCl2��Һ�������ᡢ���ᡢ�����ơ���������������ɫ��Һ���� |
�鿴�𰸺ͽ���>>
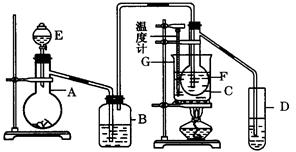
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

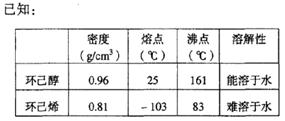
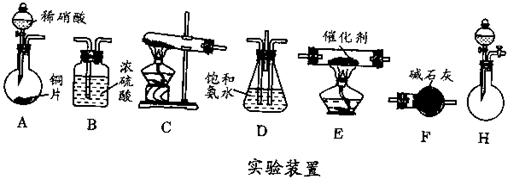
 �ڽ��롣����ʱҪ������ʯ�ң�Ŀ���� ��
�ڽ��롣����ʱҪ������ʯ�ң�Ŀ���� ��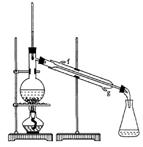
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������������ϼ��Ƚ������ȷ�Ӧ�� |
| B������������ˮ�����ȼ����Ӻ��ټ����������Ƶ�Cu(OH)2�Ϳ�ʵ�ֽ�����ˮ�⣬�������Ƶ�Cu(OH)2�������ǵ�ˮ����� |
| C��ֻ��������Ȼ�̼��Һ���ܼ�����������ϩ |
| D�����Ҵ���Ũ�����ȥ���������е��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | �������� | ���� |
| A | ���Ȼ�������������������ˮ�������Ȼ�����Һ | ����Fe3+��ˮ�� |
| B | ij��Һ�м���ϡ���ᣬ�ų���ɫ��ζ���壬������ͨ�����ʯ��ˮ��ʯ��ˮ����� | ����Һ�д���CO32�� |
| C | ��ˮ�м���ױ���������ã���ˮ����ɫ | �ױ�����ˮ������ȡ����Ӧ |
| D | �����ھƾ��ƻ����ϼ����ۻ��������� | �������۵�ߣ���ס���ڵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ���ⶨij��֭��ά����C�ĺ������仯ѧ����ʽ������ʾ������˵����ȷ����
���ⶨij��֭��ά����C�ĺ������仯ѧ����ʽ������ʾ������˵����ȷ����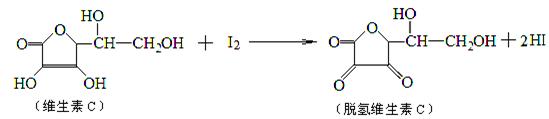
| A��������ӦΪ��ԭ��Ӧ | B���ζ�ʱӦ��������ƿ |
| C���ζ�ʱ���õ�����Һ��ָʾ�� | D���ζ�ʱ���÷�̪��ָʾ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com