


 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1mol��Լ����6.02��1023���� |
| B������ӵ���������ֵԼ����6.02��1023 |
| C���Ƶ�Ħ����������ֵ��һ�������Ƶ����ԭ������ |
| D������Ħ�������ָ��λ���ʵ�����������ռ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
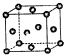 ��֪��X��Y��ZΪ������Ԫ�أ�X��Yͬ���ڣ�X��Zͬ���壬Yԭ�ӻ�̬ʱ��2p�����δ�ɶԵĵ�������࣬X�ĵͼ��������Y���ʷ��ӵĵ�������ȣ�W2+�ĺ�������Ų���ʽΪ��1S22S22P63S23P63d9��
��֪��X��Y��ZΪ������Ԫ�أ�X��Yͬ���ڣ�X��Zͬ���壬Yԭ�ӻ�̬ʱ��2p�����δ�ɶԵĵ�������࣬X�ĵͼ��������Y���ʷ��ӵĵ�������ȣ�W2+�ĺ�������Ų���ʽΪ��1S22S22P63S23P63d9���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
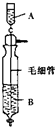 ��Inorganic Syntheses�������ϳɣ�һ���У���һ��ͼ��ʾ��װ�ã������Ʊ�ij�ָ���Ĵ������壮��װ������װ��ҩƷ��ȷ���ǣ�������
��Inorganic Syntheses�������ϳɣ�һ���У���һ��ͼ��ʾ��װ�ã������Ʊ�ij�ָ���Ĵ������壮��װ������װ��ҩƷ��ȷ���ǣ�������| A��A��װŨ���ᣬB��װŨ���� |
| B��A��װŨ���ᣬB��װŨ���� |
| C��A��װ��������Ũ��Һ��B��װŨ��ˮ |
| D��A��װŨ��ˮ��B��װ��������Ũ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
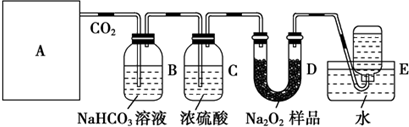
�鿴�𰸺ͽ���>>
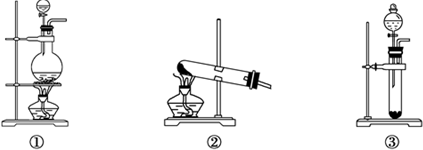
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
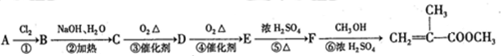 ��
��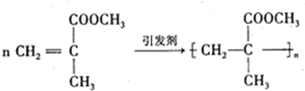
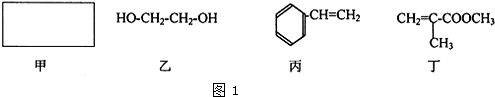
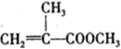 ���л������ĵ��壮��������������ϩ�����ͬ���칹�����
���л������ĵ��壮��������������ϩ�����ͬ���칹�����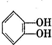 ����
����
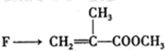
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c(H+) |
| c(CH3COOH) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ȼ��ƺ������ |
| B�����ۺ�п�� |
| C���Ȼ��غ�̼��� |
| D��̼��狀��Ȼ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com