
ij��ѧ����0.1 mol��L��1�����0.1 mol��L��1NaOH��Һ��Ӧ�ⶨ���ζ����ߣ��ס��ҡ�������ͬѧ��ƿ�е���Һ��ȡ�����Ϊ20.00mL�������õ��Լ���ȫ��ͬ������ʵ�����õ����ݻ��Ƶ����߷ֱ���ͼ��a��b��c��ʾ������˵���������

A������ͬѧ�����ϵIJ������ڵζ��յ㸽�����Ժͼ�¼pH�ļ��̫��
B������ͬѧ�ζ�ʱѡ�õĵζ���Ϊ��ʽ�ζ���
C���Һͱ�����ͬѧ�IJ��������ڲ����ʧ��
D������ͬѧ�������ô���Һ��ϴ����ƿ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����ϵ��Ĵ��ʼ컯ѧ�Ծ��������棩 ���ͣ�ʵ����
��ˮú���ǹ�ҵ�ϳɰ���ԭ����������Ҫ�ɷ���H2��CO��CO2��N2��H2O ��g������ˮú���������в���ת��Ϊ �ϳɰ���ԭ�ϡ����������գ�
�ϳɰ���ԭ�ϡ����������գ�
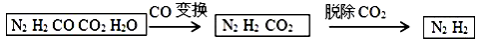
��1����ˮú�������������⡣����ˮú����Ʒͨ�� ��Һ�У���д�Լ����ƣ������� ������֤����������ڡ�
��2��д����ˮú����ͭ����ʵ��CO�任�ķ�Ӧ����ʽ ������ˮú����V��H2��: V��CO��: V��N2��=9��7��4����CO�任��������У� V��H2��: V��N2��=__________��
V��H2��: V��N2��=__________��
��3����Һ���շ����ѳ�������̼�ķ���֮һ����һ�����˶�����̼������������Һ��Ϊ�ⶨ����Һ��ɣ�����˫ָʾ�������еζ���
���裺����Һ����ȡV0mL�ĸ���Һ����ƿ�У��ȵ���1-2�η�̪��ָʾ�����ζ��ﵽ�յ�ʱ��ȥc mol��L-1�ı�������ҺVlmL����ʱ������Һ�е���1-2��__________��ָʾ�����ﵽ�ζ��յ�ʱ����ȥV2mL��������Һ��
�ڶ��εζ��ﵽ�յ�ʱ��������______________________��
���ݴ�����ͨ��Vl��V2����Դ�С���Զ���Ҳ�ɶ����ж���Һ����ɡ�
��V1>V2��д����ʱ�����ʵĻ�ѧʽ_______________��
��2V1=V2��д����ʱ��Һ������Ũ���ɴ�С��˳��_________________��
���ۣ���V1<V2���ڵ�һ�εζ�ʱδ�ñ�������ϴ��ʽ�ζ��ܣ��ڶ��εζ�ǰ���ֲ������˴�����____________���ѧʽ��Ũ��ƫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶������л�ѧ���������棩 ���ͣ�ʵ����
ijʵ��С����0.50mol/LNaOH��Һ��0.50mol/L������Һ�����к��ȵIJⶨ��
������0.50mol/LNaOH��Һ
��ʵ���д�ԼҪʹ��245mLNaOH��Һ��������Ҫ����NaOH����___________g
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ������ͼ��ʾ��
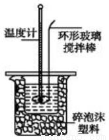
ȡ50mLNaOH��Һ��30mL������Һ����ʵ�飬ʵ���������±���
��1��д���÷�Ӧ���Ȼ�ѧ����ʽΪ___________���к���Ϊ57.3kJ/mol����
��2��������д�±��еĿհף�
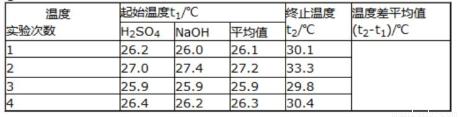
�ڽ�����Ϊ0.50mol/LNaOH��Һ��0.50mol/L������Һ���ܶȶ���1g/cm3���кͺ�������Һ�ı�����c=4.18J/��g•�棩�����к��ȡ�H=___________��ȡС�����һλ����
������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ�������___________������ĸ��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶������л�ѧ���������棩 ���ͣ������
�����½�a mL����һԪ��ֱ��NaOH��Һ�������ϣ�ʵ���������£�
��� | c��һԪ�ᣩ | c��NaOH�� / mol��L��1 | �����Һ��pH |
�� | c��HX��=0��1 mol��L��1 | 0.1 | pH = 10 |
�� | c��HY��=0��1 mol��L��1 | 0.1 | pH = 7 |
�� | c��HZ��=0��1 mol��L��1 | 0.1 | pH = 9 |
��1�����ݱ������ݱȽ�HX��HY��HZ�������������ǿ������˳��Ϊ_________������ʵ�鷢����Ӧ�����ӷ���ʽΪ ��������Һ����ˮ�������c��OH����= mol��L��1��
��2������ʵ�鷴Ӧ�����е�pH�仯��������ͼ��
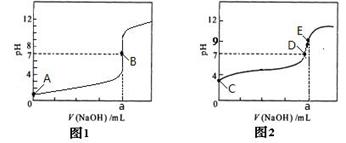
�ٱ�ʾ����ʵ���pH�仯������________________���ͼ1����ͼ2����
��������ͼ�б�ʾ��Һǡ����ȫ��Ӧ�ĵ���________________��
��3�������£���pH=3������aL�ֱ�������������Һ��ϣ�������Һ�������ԡ�
��Ũ��Ϊ1.0��10��3 mol��L��1�İ�ˮb L
��c��OH������1.0��10��3 mol��L��1�İ�ˮc L
��c��OH������1.0��10��3 mol��L��1������������Һd L����a��b��c��d�Ĵ�С��ϵ��_________________��
��4�������£�ȡpH=2������ʹ�����Һ��100mL�������зֱ����������Zn������Ӧ����������Һ��pH�仯��ͼ��ʾ����ͼ�б�ʾ������Һ��pH�仯���ߵ���__________���A����B�������������м����Zn����Ϊm1��������Һ�м����Zn����Ϊm2����m1__________m2�����������=������������
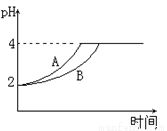
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶������л�ѧ���������棩 ���ͣ�ѡ����
�����£�������Һ������Ũ�ȹ�ϵ��ȷ����
A��Na2S��Һ��c��Na+��>c��HS-��>c��OH-��>c��H2S��
B��Na2C2O4��Һ��c��OH-��=c��H+��+c��HC2O4-��+2c��H2C2O4��
C��Na2CO3��Һ��c��Na+��+c��H+��=2c��CO32-��+c��OH-��
D��CH3COONa��CaCl2�����Һ��c��Na+��+c��Ca2+��=c��CH3COO-��+c��CH3COOH��+2c��Cl-��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶������л�ѧ���������棩 ���ͣ�ѡ����
ij�ܱ������з�����Ӧ��X��g��+3Y��g�� 2Z��g�� ��H<0����ͼ��ʾ�÷�Ӧ�����ʣ�v����ʱ�䣨t���仯�Ĺ�ϵ��t2��t3��t5ʱ��������������ı䣬����û�иı�����ʵij�ʼ������������˵������ȷ����
2Z��g�� ��H<0����ͼ��ʾ�÷�Ӧ�����ʣ�v����ʱ�䣨t���仯�Ĺ�ϵ��t2��t3��t5ʱ��������������ı䣬����û�иı�����ʵij�ʼ������������˵������ȷ����
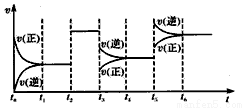
A��t2ʱ�����˴��� B��T3ʱƽ����ƶ���ʹ��ѧƽ�ⳣ����С
C��t5ʱ������ѹǿ D��T6ʱ�ﵽƽ���Ӧ���ת�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶������л�ѧ���������棩 ���ͣ�ѡ����
N2H4��һ�ָ�Ч���Ļ��ȼ�ϡ���֪0.5molN2H4��g����ȫȼ�����ɰ�������̬ˮʱ���ų�267kJ�������������Ȼ�ѧ��������ȷ����
A��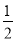 N2H4��g��+
N2H4��g��+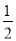 O2��g��=
O2��g��=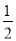 N2��g��+H2O��g�� ��H=+267kJ/mol
N2��g��+H2O��g�� ��H=+267kJ/mol
B��N2H4��g��+O2��g��=N2��g��+2H2O��l�� ��H=-534kJ/mol
C��N2H4��g��+O2��g��=N2��g��+2H2O��g�� ��H=+534kJ/mol
D��N2H4��g��+O2��g��=N2��g��+2H2O��g�� ��H=-534kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶����¿�����ѧ���������棩 ���ͣ�ѡ����
��25��ʱ����amol/L�İ�ˮ��0.01mol/L��HCl��Һ�������ϣ���Ӧ����Һ�����ԣ�����˵������ȷ���ǣ�������Һ��Ϻ�����ı仯��
A����Һ��c��NH4+��=c��Cl����
B����ʱˮ�ĵ���̶����
C����ʱ��Һ��NH3��H2O�����ʵ���Ũ��Ϊ1/2[��a-0.01��]mol/L
D���ú�a �Ĵ���ʽ NH3��H2O�ĵ���ƽ�ⳣ��Kb��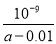 ��mol/L��
��mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶������л�ѧ���������棩 ���ͣ�ѡ����
������ʵ�����û�ѧƽ���ƶ�ԭ�����͵���
A���������Ƶ���ˮʱ����Һ��PH����
B������ѹǿ��������SO2��O2��Ӧ����SO3
C������Ũ��ˮ�����������ƹ��������ȡ��
D��500�����ұȳ����¸������ںϳɰ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com