
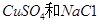 �Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ����
�Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ����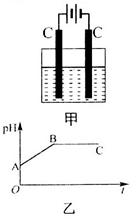
A���Ǹû����Һ�е� ������A����Һ��pHֵС��B�� ������A����Һ��pHֵС��B�� |
B��AB�߶���BC�߶��������Ϸ����ķ�Ӧ����ͬ�ļ���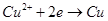 |
| C��BC�����������������������֮��Ϊ2��1 |
| D�����������Ĺ����л������������ɫ��Cu��OH��2���� |


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �� �� | ��ʼ���� | ������ȫ |
| Fe(OH)3 | 2.7 | 3.7 |
| Fe(OH)2 | 7.6 | 9.6 |
| Mn(OH)2 | 8.3 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
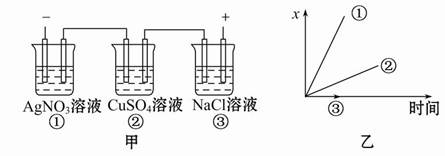
| A������������������������ı仯 |
| B���������������������� |
| C���������������������� |
| D�����缫�Ϸŵ�����������ı仯 |
�鿴�𰸺ͽ���>>
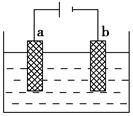
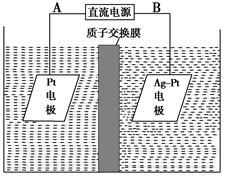
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
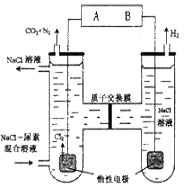
| A��BΪ �� Դ ���� �� |
| B���������У������Ҹ�����PH���ֲ��� |
| C���������У������ɵ�ԴB����������Һ���ص���ԴA�� |
| D���ұ߷����ĵ缫��ӦʽΪ��2H2O +2e��= H2��+ 2OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

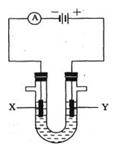
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
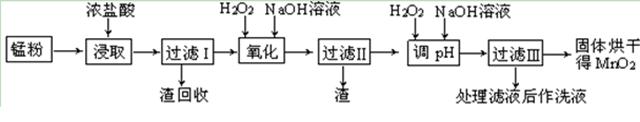
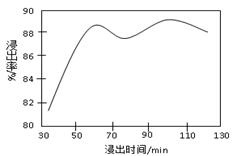
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
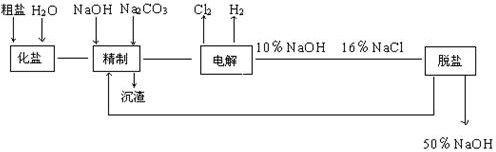
 �����ϸߣ��������ӱ�ʽ����ȥSO
�����ϸߣ��������ӱ�ʽ����ȥSO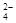 ���ñ��Լ���������ѡ��A.B.c����ѡ�۷֣�
���ñ��Լ���������ѡ��A.B.c����ѡ�۷֣� 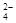 �������Լ��ĺ���˳��Ϊ��ѡ��a��b��c��ѡ�۷֣�
�������Լ��ĺ���˳��Ϊ��ѡ��a��b��c��ѡ�۷֣�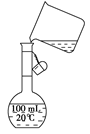
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com