



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| PH | 6.5”«8.5 |
| Ca2+”¢Mg2+×ÜÅØ¶Č | £¼0.0045mol/L |
| Ļø¾ś×ÜŹż | £¼100øö/mL |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø17·Ö£©ĪŅ¹ś¹ę¶ØŅūÓĆĖ®ÖŹĮæ±ź×¼±ŲŠė·ūŗĻĻĀĮŠŅŖĒó£ŗ
| PH | 6£®5~8£®5 |
| Ca2£«”¢Mg2£«×ÜÅØ¶Č | £¼0.0045mol/L |
| Ļø¾ś×ÜŹż | £¼100øö/mL |
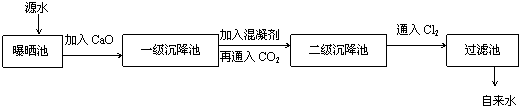
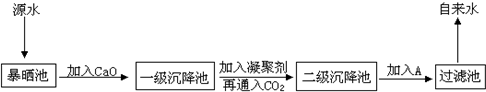
ij×ŪŗĻŹµ¼ł»ī¶ÆŠ”×éµ½×ŌĄ“Ė®³§²Ī¹Ū£¬ĮĖ½āµ½Ō“Ė®“¦Ąķ³É×ŌĄ“Ė®µÄ¹¤ŅÕĮ÷³ĢŹ¾ŅāĶ¼ČēĻĀ£ŗ
£Ø1£©Ō“Ė®ÖŠŗ¬ÓŠCa2£«”¢Mg2£«”¢HCO3-”¢Cl-µČ£¬¼ÓČėCaOŗó·¢ÉśČōøÉøö»Æѧ·“Ó¦£¬ĒėŠ“³öĘäÖŠČĪŅāŅ»øö·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ_____________________________________
£Ø2£©¼ÓČėŠõÄż¼ĮæÉŅŌ³żČ„ĘäÖŠµÄŠüø”¹ĢĢåæÅĮ££¬øĆ¹ż³ĢŹĒ____________________£ØĢīŠņŗÅ£©
¢Ł Ö»ÓŠĪļĄķ¹ż³Ģ£¬ĪŽ»Æѧ¹ż³Ģ
¢Ś Ö»ÓŠ»Æѧ¹ż³Ģ£¬ĪŽĪļĄķ¹ż³Ģ
¢Ū ¼ČÓŠĪļĄķ¹ż³Ģ£¬ÓÖÓŠ»Æѧ¹ż³Ģ
£Ø3£©FeSO4”¤7H2OŹĒ³£ÓƵĊõÄż¼Į£¬¼ÓČėŗó×īÖÕÉś³ÉŗģŗÖÉ«½ŗד³Įµķ£¬ŌņÕāÖÖ³ĮµķŹĒ___________£ØĢī»ÆѧŹ½£©
£Ø4£©ĶØČė¶žŃõ»ÆĢ¼µÄÄæµÄŹĒ__________ŗĶ___________”£
£Ø5£©ĪļÖŹAµÄ×÷ÓĆŹĒ_________£¬ŌņAæÉŅŌŃ”ŌńĻĀĮŠĪļÖŹÖŠµÄ__________£ØĢīŠņŗÅ£©
¢ŁClO2 ¢ŚSO2 ¢ŪŅŗĀČ ¢ÜCa(ClO2)2 ¢ŻÅØĮņĖį
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģŗÓÄĻŹ”½¹×÷ŹŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø17·Ö£©ĪŅ¹ś¹ę¶ØŅūÓĆĖ®ÖŹĮæ±ź×¼±ŲŠė·ūŗĻĻĀĮŠŅŖĒó£ŗ
|
PH |
6£®5~8£®5 |
|
Ca2£«”¢Mg2£«×ÜÅØ¶Č |
£¼0.0045mol/L |
|
Ļø¾ś×ÜŹż |
£¼100øö/mL |
ij×ŪŗĻŹµ¼ł»ī¶ÆŠ”×éµ½×ŌĄ“Ė®³§²Ī¹Ū£¬ĮĖ½āµ½Ō“Ė®“¦Ąķ³É×ŌĄ“Ė®µÄ¹¤ŅÕĮ÷³ĢŹ¾ŅāĶ¼ČēĻĀ£ŗ
 [Ą“Ō“:ZXXK]
[Ą“Ō“:ZXXK]
£Ø1£©Ō“Ė®ÖŠŗ¬ÓŠCa2£«”¢Mg2£«”¢HCO3-”¢Cl-µČ£¬¼ÓČėCaOŗó·¢ÉśČōøÉøö»Æѧ·“Ó¦£¬ĒėŠ“³öĘäÖŠČĪŅāŅ»øö·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ_____________________________________
£Ø2£©¼ÓČėŠõÄż¼ĮæÉŅŌ³żČ„ĘäÖŠµÄŠüø”¹ĢĢåæÅĮ££¬øĆ¹ż³ĢŹĒ____________________£ØĢīŠņŗÅ£©
¢Ł Ö»ÓŠĪļĄķ¹ż³Ģ£¬ĪŽ»Æѧ¹ż³Ģ
¢Ś Ö»ÓŠ»Æѧ¹ż³Ģ£¬ĪŽĪļĄķ¹ż³Ģ
¢Ū ¼ČÓŠĪļĄķ¹ż³Ģ£¬ÓÖÓŠ»Æѧ¹ż³Ģ
£Ø3£©FeSO4”¤7H2OŹĒ³£ÓƵĊõÄż¼Į£¬¼ÓČėŗó×īÖÕÉś³ÉŗģŗÖÉ«½ŗד³Įµķ£¬ŌņÕāÖÖ³ĮµķŹĒ___________£ØĢī»ÆѧŹ½£©
£Ø4£©ĶØČė¶žŃõ»ÆĢ¼µÄÄæµÄŹĒ__________ŗĶ___________”£
£Ø5£©ĪļÖŹAµÄ×÷ÓĆŹĒ_________£¬ŌņAæÉŅŌŃ”ŌńĻĀĮŠĪļÖŹÖŠµÄ__________£ØĢīŠņŗÅ£©
¢ŁClO2 ¢ŚSO2 ¢ŪŅŗĀČ ¢ÜCa(ClO2)2 ¢ŻÅØĮņĖį
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com