
���� ��1��ij�¶��£�Kw=1��10-12�� mol•L-1��2�����¶���ˮ�м���ϡ�������Һ�д���c��H+��/c��OH-��=1��1010������Һ��c��H+��=0.1mol/L������Һ��ˮ�������c��OH-��=$\frac{1{0}^{-12}}{0.1}$mol/L=10-11 mol/L������Һ��ˮ�������c��H+��������Һ��c��OH-����
��2��pH=3��HA��Һ�У�c��HA����0.001mol/L��pH=11��NaOH��Һ��c��NaOH��=0.001mol/L��
A�������£������Һ�����ԣ�������Һ��c��OH-��=c��H+��=10-7mol/L��
B��������ߵ������ȣ���n��HA����n��NaOH���������Һ�����Ի����ԣ�
C������Ӧ����Һ�����ԣ���V1һ����һ������V2��
D����HAΪ���ᣬ�������ϳ����ԣ���ʼ��ԣ�V1һ��С��V2��
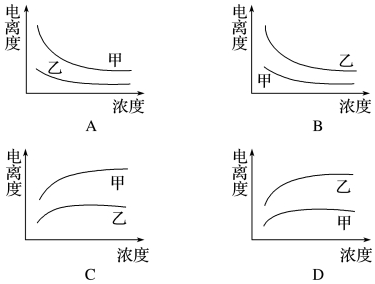
��3�������ᣨ�ס�Ka=1.8��10-5����һ�����ᣨ�ҡ�Ka=1.4��10-3����֪�������KaС�������������������Ũ��ʱ����ǿ�ĵ���ȴ������Ũ��Խ��������ԽС��
��4��ǿ������ʵĸ��������ǵ���̶ȣ����ֵ���ĵ������������ʣ�HCl��ǿ����ʣ�ֻҪ֤����Һ�д��ڴ������ƽ�����֤����������С�����ᣮ
��� �⣺��1��ij�¶��£�Kw=1��10-12�� mol•L-1��2�����¶���ˮ�м���ϡ�������Һ�д���c��H+��/c��OH-��=1��1010������Һ��c��H+��=0.1mol/L������Һ��ˮ�������c��OH-��=$\frac{1{0}^{-12}}{0.1}$mol/L=10-11 mol/L������Һ��ˮ�������c��H+��������Һ��c��OH-��=10-11 mol/L��
�ʴ�Ϊ��1��10-11��
��2��pH=3��HA��Һ�У�c��HA����0.001mol/L��pH=11��NaOH��Һ��c��NaOH��=0.001mol/L��
A������ҺM�����ԣ���c��H+��=c��OH-��=1��10-7mol•L-1����c��H+��+c��OH-��=2��10-7mol•L-1����A��ȷ��
B����V1=V2������HA��ǿ��δ֪����Ӧ����Һ������Բ���ȷ������ҺM��pH��һ������7����B����
C����HAΪ���ᣬ�������ϣ���ҺҲ���ܳ����ԣ���V1��һ������V2����C����
D����HAΪ���ᣬ�������ϳ����ԣ���ʼ��ԣ�V1һ��С��V2����HAΪǿ�ᣬ��Ӧ��ʼ��ԣ���V1һ��С��V2����D��ȷ��
��ѡ��AD��
��3�������ᣨ�ס�Ka=1.8��10-5����һ�����ᣨ�ҡ�Ka=1.4��10-3����֪�������KaС�����������������
��ͼ��֪��������ΪŨ�ȣ�������Ϊ����ȣ����Ũ��ʱ����ǿ�ĵ���ȴ��ҵ��������Ϸ������ų�A��C��
�����Ũ��Խ��������ԽС�������ס��Ҿ���Ũ�ȵ�������½������ų�D����Ȼֻ��B���ϣ�
��ѡB��
��4��A�����������pH=4������ʹ���ϡ�ͳ�pH=5����Һ�������������ˮ���Ķ��٣���������ˮ���������ᣬ˵�������д��ڵ���ƽ�⣬���������С�����ᣬ��Aѡ��
B������������pH������ʹ����зֱ����ͬ������Ӧ���ι��壬����Һ��pH���ޱ仯�����������ƴ�����뵼������ҺpH������֤����������С�����ᣬ��Bѡ��
C������������Ũ�ȵ�����ʹ���ֱ��������п�ۣ����������������������������������������ʵ��������ȣ�������ǿ���أ����Բ���֤����������С�����ᣬ��C��ѡ��
D���õ��������Ũ�ȵ�����ʹ�����������ʵ�飬���ݵ��ݵ������̶�ȷ����Һ������Ũ�ȣ��Ӷ�ȷ������ǿ������Dѡ��
��ѡABD��
���� ���⿼���������Һ�����жϡ���������жϡ�ˮ�ĵ����֪ʶ�㣬���ؿ���ѧ�������ж���������ȷ������ʵ����ص㡢ˮ����Ӱ�������ǽⱾ��ؼ���ע��������Һ��ˮ�����������Ũ�ȼ��㷽������Ŀ�ѶȲ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��������ŷŹ����Ķ�����̼�����ȫ�������ů����Ҫ���أ����Զ�����̼�IJ����������ҹ���Դ�����һ����Ҫս�Է���
��������ŷŹ����Ķ�����̼�����ȫ�������ů����Ҫ���أ����Զ�����̼�IJ����������ҹ���Դ�����һ����Ҫս�Է��� ����������C02ͨ��������Һ�У�������Һ����ǵ���ce��
����������C02ͨ��������Һ�У�������Һ����ǵ���ce���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ƿ���������ȵķ�Ӧ�� | |
| B�� | �����£����ܽ�Ũ����ʢ������Ͱ�� | |
| C�� | ��50mL��Ͳ������0.1000mol•L-1̼������Һ | |
| D�� | ������ˮ��ʪ����ֽ����Һ��pH��һ����ʹ���ƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������������䣬�����¶ȣ�H2SŨ�����ӣ������÷�Ӧ�����ȷ�Ӧ | |
| B�� | ͨ��CO������Ӧ���������� | |
| C�� | ��ӦǰH2S���ʵ���Ϊ7 mol | |
| D�� | CO��ƽ��ת����Ϊ80% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ˮ�ĵ���ƽ���У�c��H+����c��OH-���Ĺ�ϵ��ͼ��ʾ��
��ˮ�ĵ���ƽ���У�c��H+����c��OH-���Ĺ�ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ܢ� | B�� | �ڢۢ� | C�� | �٢ۢ� | D�� | �٢ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com