
ПтТ»ИЭ»эІ»ұдөДГЬұХИЭЖчЦРідИлТ»¶ЁБҝAәНBЈ¬·ўЙъ·ҙУҰЈәxA(g)Ј«2B(s) yC(g)ЎЎҰӨHЈј0ЎЈФЪТ»¶ЁМхјюПВЈ¬ИЭЖчЦРAЎўCөДОпЦКөДБҝЕЁ¶ИЛжКұјдұд»ҜөДЗъПЯИзНјЛщКҫЎЈЗл»ШҙрПВБРОКМвЈә
yC(g)ЎЎҰӨHЈј0ЎЈФЪТ»¶ЁМхјюПВЈ¬ИЭЖчЦРAЎўCөДОпЦКөДБҝЕЁ¶ИЛжКұјдұд»ҜөДЗъПЯИзНјЛщКҫЎЈЗл»ШҙрПВБРОКМвЈә

(1)УГAөДЕЁ¶Иұд»ҜұнКҫёГ·ҙУҰ0Ў«10 minДЪөДЖҪҫщ·ҙУҰЛЩВКv(A)ЈҪ________ЎЈ
(2)ёщҫЭНјКҫҝЙИ·¶ЁxЎГyЈҪ________ЎЈ
(3)0Ў«10 minИЭЖчДЪС№Зҝ________(МоЎ°ұдҙуЎұЎўЎ°І»ұдЎұ»тЎ°ұдРЎЎұ)ЎЈ
(4)НЖІвөЪ10 minТэЖрЗъПЯұд»ҜөД·ҙУҰМхјюҝЙДЬКЗ________Ј»өЪ16 minТэЖрЗъПЯұд»ҜөД·ҙУҰМхјюҝЙДЬКЗ________ЎЈ
ўЩјхС№ЎЎўЪФцҙуAөДЕЁ¶ИЎЎўЫФцҙуCөДБҝЎЎўЬЙэОВ ўЭҪөОВЎЎўЮјУҙЯ»ҜјБ
(5)ИфЖҪәвўсөДЖҪәвіЈКэОӘK1Ј¬ЖҪәвўтөДЖҪәвіЈКэОӘK2Ј¬ФтK1________K2(МоЎ°ЈҫЎұЎўЎ°ЈҪЎұ»тЎ°ЈјЎұ)ЎЈ
ЎЎ(1)0.02 mol/(LЎӨmin)ЎЎ(2)1ЎГ2ЎЎ(3)ұдҙу (4)ўЬўЮЎЎўЬЎЎ(5)Јҫ
ЎҫҪвОцЎҝЎЎ(1)AКЗ·ҙУҰОпЈ¬10 minДЪЖдЕЁ¶ИјхРЎБҝОӘ(0.45Јӯ0.25)molЎӨLЈӯ1Ј¬ФтЖҪҫщ·ҙУҰЛЩВККЗ0.02 mol/(LЎӨmin)ЎЈ(2)10 minКұCөДЕЁ¶ИФцјУ0.40 molЎӨLЈӯ1Ј¬ФтУлAөДЕЁ¶Иұд»ҜЦ®ұИКЗ2ЎГ1Ј¬№КxЎГyЈҪ1ЎГ2ЎЈ(3)0Ў«10 minДЪCөДЕЁ¶ИФцјУөДБҝұИAөДЕЁ¶ИјхРЎөДБҝҙуЈ¬BКЗ№ММеЈ¬ЛщТФС№ЗҝұдҙуЎЈ(4)ёщҫЭНјПсЦӘ10 minКұ·ҙУҰЛЩВКјУҝмЈ¬·ЦОцҝЙЦӘ10 minКұёДұдөДМхјюҝЙДЬКЗЙэОВ»тјУҙЯ»ҜјБЈ»12Ў«16 minЈ¬·ҙУҰҙҰУЪЖҪәвЧҙМ¬Ј¬16 minәуAөДЕЁ¶ИЦрҪҘФцҙуЈ¬CөДЕЁ¶ИЦрҪҘјхРЎЈ¬20 minКұҙпөҪРВЖҪәвЈ¬·ЦОцҝЙЦӘ16 minКұёДұдөДМхјюҝЙДЬКЗЙэОВЎЈ(5)ЙэОВЖҪәвЧуТЖЈ¬KјхРЎЎЈ



| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013-2014С§ДкёЯҝј»ҜС§¶юВЦёҙП°ҪӯЛХЧЁУГ ёЯҝјДЈДвСЭБ·2Б·П°ҫнЈЁҪвОц°жЈ© МвРНЈәСЎФсМв
УГNa2SO3ИЬТәОьКХБтЛб№ӨТөОІЖшЦРөД¶юСх»ҜБтЈ¬Ҫ«ЛщөГөД»мәПТәҪшРРөзҪвСӯ»·ФЩЙъЈ¬ХвЦЦРВ№ӨТХҪРФЩЙъСӯ»·НСБт·ЁЎЈЖдЦРТхЎўСфАлЧУҪ»»»ДӨЧйәПСӯ»·ФЩЙъ»ъАнИзНјЛщКҫЈ¬ФтПВБРУР№ШЛө·ЁЦРІ»ХэИ·өДКЗЈЁЎЎЎЎЈ©ЎЈ

AЈ®XОӘЦұБчөзФҙөДёәј«Ј¬YОӘЦұБчөзФҙөДХэј«
BЈ®НјЦРөДb>a
CЈ®Сфј«ЗшpHФцҙу
DЈ®ёГ№эіМЦРөДІъЖ·ЦчТӘОӘH2SO4әНH2
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013-2014С§ДкёЯҝј»ҜС§¶юВЦёҙП°ҪӯЛХЧЁУГ өЪ9ҪІ·ЗҪрКфФӘЛШј°Жд»ҜәПОпБ·П°ҫнЈЁҪвОц°жЈ© МвРНЈәСЎФсМв
ПВБРЛө·Ё·ыәПВМЙ«»ҜС§ЛјПлөДКЗ(ЎЎЎЎ)ЎЈ
AЈ®¶Ф№ӨТөЙъІъПхЛбІъЙъөД·ПЖшЎў·ПЛ®ҪшРРСПёсҙҰАн
BЈ®АыУГёщБцҫъ№МөӘТФјхЙЩөӘ·КЙъІъі§
CЈ®КөСйКТЦЖұёNO2КұФЪНЁ·зічДЪҪшРР
DЈ®ЙъІъБтЛбөД№Өі§ЦЦІЭЎўЦЦКчЈ¬К№ЖдіЙОӘЎ°»ЁФ°КҪ№Өі§Ўұ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013-2014С§ДкёЯҝј»ҜС§¶юВЦёҙП°ҪӯЛХЧЁУГ өЪ7ҪІЛ®ИЬТәЦРөДАлЧУЖҪәвБ·П°ҫнЈЁҪвОц°жЈ© МвРНЈәМоҝХМв
25 ЎжКұЈ¬өзАлЖҪәвіЈКэЈә
»ҜС§КҪ | CH3COOH | H2CO3 | HClO |
өзАлЖҪәвіЈКэ | 1.8ЎБ10Јӯ5 | K1 4.3ЎБ10Јӯ7 K2 5.6ЎБ10Јӯ11 | 3.0ЎБ10Јӯ8 |
»ШҙрПВБРОКМвЈә
(1)ОпЦКөДБҝЕЁ¶ИОӘ0.1 molЎӨLЈӯ1өДПВБРЛДЦЦОпЦКЈәa.Na2CO3Ј¬b.NaClOЈ¬c.CH3COONaЈ¬d.NaHCO3Ј»pHУЙҙуөҪРЎөДЛіРтКЗ______________(МоұаәЕ)Ј»
(2)іЈОВПВ0.1 molЎӨLЈӯ1өДCH3COOHИЬТәјУЛ®ПЎКН№эіМЈ¬ПВБРұнҙпКҪөДКэҫЭТ»¶ЁұдРЎөДКЗ________Ј»ЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎ
AЈ®c(HЈ«) B. CЈ®c(HЈ«)ЎӨc(OHЈӯ) D.
CЈ®c(HЈ«)ЎӨc(OHЈӯ) D.
(3)Ме»эОӘ10 mL pHЈҪ2өДҙЧЛбИЬТәУлТ»ФӘЛбHXИЬТә·ЦұрјУЛ®ПЎКНЦБ1 000 mLЈ¬ПЎКН№эіМpHұд»ҜИзНјЈәФтHXөДөзАлЖҪәвіЈКэ________(МоЎ°ҙуУЪЎұЎўЎ°өИУЪЎұ»тЎ°РЎУЪЎұ)ҙЧЛбөДЖҪәвіЈКэЈ»АнУЙКЗ__________________________________________Ј¬ПЎКНәуЈ¬HXИЬТәЦРЛ®өзАліцАҙөДc(HЈ«)________ҙЧЛбИЬТәЛ®өзАліцАҙc(HЈ«)(МоЎ°ҙуУЪЎұЎўЎ°өИУЪЎұ»тЎ°РЎУЪЎұ)АнУЙКЗЈә____________________________________Ј»

(4)25 ЎжКұЈ¬CH3COOHУлCH3COONaөД»мәПИЬТәЈ¬ИфІвөГ»мәПТәpHЈҪ6Ј¬ФтИЬТәЦРc(CH3COOЈӯ)Јӯc(NaЈ«)ЈҪ________(МоЧјИ·КэЦө)ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013-2014С§ДкёЯҝј»ҜС§¶юВЦёҙП°ҪӯЛХЧЁУГ өЪ7ҪІЛ®ИЬТәЦРөДАлЧУЖҪәвБ·П°ҫнЈЁҪвОц°жЈ© МвРНЈәСЎФсМв
ПВБРЛө·ЁХэИ·өДКЗ(ЎЎЎЎ)ЎЈ
AЈ®0.1 molЎӨLЈӯ1өДҙЧЛбИЬТәјУЛ®ПЎКНЈ¬ јхРЎ
јхРЎ
BЈ®Ме»эЎўpHҫщПаН¬өДҙЧЛбәНСОЛбНкИ«ИЬҪвөИБҝөДГҫ·Ы(ЙЩБҝ)Ј¬әуХЯУГКұЙЩ
CЈ®ПтЛ®ЦРјУИлЙЩБҝ№ММеБтЛбЗвДЖЈ¬c(HЈ«)ФцҙуЈ¬KWұдҙу
DЈ®іЈОВПВЈ¬V1 L pHЈҪ11өДNaOHИЬТәУлV2 L pHЈҪ3өДHAИЬТә»мәПЈ¬Иф»мәПТәПФЦРРФЈ¬ФтV1ЎЬV2
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013-2014С§ДкёЯҝј»ҜС§¶юВЦёҙП°ҪӯЛХЧЁУГ өЪ6ҪІ»ҜС§·ҙУҰЛЩВКәН»ҜС§ЖҪәвБ·П°ҫнЈЁҪвОц°жЈ© МвРНЈәСЎФсМв
Т»¶ЁМхјюПВЈ¬ПтИЭ»эОӘ2 LөДГЬұХИЭЖчЦРідИл1 mol CO2әН3 mol H2Ј¬·ўЙъИзПВ·ҙУҰЈәCO2(g)Ј«3H2(g) CH3OH(g)Ј«H2O(g)Ј¬5 minәу·ҙУҰҙпөҪЖҪәвКұc(CH3OH)ОӘ0.2 molЎӨLЈӯ1ЎЈCO2(g)өДЖҪәвОпЦКөДБҝЕЁ¶Иc(CO2)УлОВ¶И№ШПөИзНјЛщКҫЎЈПВБРЛө·ЁҙнОуөДКЗ(ЎЎЎЎ)ЎЈ
CH3OH(g)Ј«H2O(g)Ј¬5 minәу·ҙУҰҙпөҪЖҪәвКұc(CH3OH)ОӘ0.2 molЎӨLЈӯ1ЎЈCO2(g)өДЖҪәвОпЦКөДБҝЕЁ¶Иc(CO2)УлОВ¶И№ШПөИзНјЛщКҫЎЈПВБРЛө·ЁҙнОуөДКЗ(ЎЎЎЎ)ЎЈ

AЈ®0Ў«5 minЈ¬CO2өДЖҪҫщ·ҙУҰЛЩВКОӘ0.04 molЎӨ(LЎӨmin)Јӯ1
BЈ®·ҙУҰCO2(g)Ј«3H2(g) CH3OH(g)Ј«H2O(g)өДҰӨHЈј0
CH3OH(g)Ј«H2O(g)өДҰӨHЈј0
CЈ®ФЪT2 ЎжКұЈ¬Иф·ҙУҰҙҰУЪЧҙМ¬DЈ¬ФтТ»¶ЁУРvХэЈјvДж
DЈ®ИфT1 ЎжЎўT2 ЎжКұөДЖҪәвіЈКэ·ЦұрОӘK1ЎўK2Ј¬ФтK1ЈҫK2
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013-2014С§ДкёЯҝј»ҜС§¶юВЦёҙП°ҪӯЛХЧЁУГ өЪ5ҪІ»ҜС§·ҙУҰУлДЬБҝБ·П°ҫнЈЁҪвОц°жЈ© МвРНЈәМоҝХМв
(1)јЧҙјКЗТ»ЦЦЦШТӘөД»Ҝ№ӨІъЖ·Ј¬ҝЙАыУГјЧҙјҙЯ»ҜНСЗвЦЖұёјЧИ©ЎЈјЧИ©УлЖшМ¬јЧҙјЧӘ»ҜөДДЬБҝ№ШПөИзНјЛщКҫЎЈ

·ҙУҰ№эіМЦРөДДЬБҝ№ШПө
ўЩјЧҙјҙЯ»ҜНСЗвЧӘ»ҜОӘјЧИ©өД·ҙУҰКЗ________(МоЎ°ОьИИЎұ»тЎ°·ЕИИЎұ)·ҙУҰЎЈ
ўЪ№эіМўсУл№эіМўтөД·ҙУҰИИКЗ·сПаН¬Јҝ____________ФӯТтКЗ__________________________________ЎЈ
ўЫРҙіцјЧҙјҙЯ»ҜНСЗвЧӘ»ҜОӘјЧИ©өДИИ»ҜС§·ҙУҰ·ҪіМКҪ________________________________ЎЈ
(2)ТСЦӘЈәўЩCH3OH(g)Ј«H2O(g)=CO2(g)Ј«3H2(g)ЎЎҰӨHЈҪЈ«49.0 kJЎӨmolЈӯ1
ўЪCH3OH(g)Ј«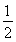 O2(g)=CO2(g)Ј«2H2(g)ЎЎҰӨHЈҪЈӯ192.9 kJЎӨmolЈӯ1
O2(g)=CO2(g)Ј«2H2(g)ЎЎҰӨHЈҪЈӯ192.9 kJЎӨmolЈӯ1
ПВБРЛө·ЁХэИ·өДКЗ________ЎЈ

AЈ®CH3OHЧӘұдіЙH2өД№эіМТ»¶ЁТӘОьКХДЬБҝ
BЈ®ўЩ·ҙУҰЦРЈ¬·ҙУҰОпөДЧЬДЬБҝёЯУЪЙъіЙОпөДЧЬДЬБҝ
CЈ®ёщҫЭўЪНЖЦӘ·ҙУҰЈәCH3OH(l)Ј«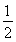 O2(g)=CO2(g)Ј«2H2(g)өДҰӨHЈҫЈӯ192.9 kJЎӨmolЈӯ1
O2(g)=CO2(g)Ј«2H2(g)өДҰӨHЈҫЈӯ192.9 kJЎӨmolЈӯ1
DЈ®·ҙУҰўЪөДДЬБҝұд»ҜИзНјЛщКҫ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013-2014С§ДкёЯҝј»ҜС§¶юВЦёҙП°ҪӯЛХЧЁУГ өЪ4ҪІОпЦКҪб№№ФӘЛШЦЬЖЪВЙБ·П°ҫнЈЁҪвОц°жЈ© МвРНЈәСЎФсМв
јёЦЦ¶МЦЬЖЪФӘЛШөДФӯЧУ°лҫ¶ј°ЦчТӘ»ҜәПјЫИзПВұнЈә
ФӘЛШҙъәЕ | X | Y | Z | W |
ФӯЧУ°лҫ¶/10Јӯ12 m | 160 | 110 | 70 | 66 |
ЦчТӘ»ҜәПјЫ | Ј«2 | Ј«5ЎўЈ«3ЎўЈӯ3 | Ј«5ЎўЈ«3ЎўЈӯ3 | Јӯ2 |
ПВБРРрКцХэИ·өДКЗ(ЎЎЎЎ)ЎЈ
AЈ®АлЧУ°лҫ¶ЈәWЈјX
BЈ®ЖшМ¬Зв»ҜОпөДОИ¶ЁРФЈәZЈҫW
CЈ®»ҜәПОпX3Z2ЦРјИә¬УРАлЧУјьУЦә¬УР№ІјЫјь
DЈ®ЧоёЯјЫСх»ҜОп¶ФУҰЛ®»ҜОпөДЛбРФЈәZЈҫY
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013-2014С§ДкёЯҝј»ҜС§¶юВЦёҙП°ҪӯЛХЧЁУГ өЪ15ҪІКөСй»ҜС§Б·П°ҫнЈЁҪвОц°жЈ© МвРНЈәКөСйМв
БтЛбСЗМъп§[(NH4)2SO4ЎӨFeSO4ЎӨ6H2O]ОӘЗіВМЙ«ҫ§МеЈ¬ТЧИЬУЪЛ®Ј¬І»ИЬУЪҫЖҫ«Ј¬ФЪЛ®ЦРөДИЬҪв¶ИұИFeSO4»т(NH4)2SO4¶јТӘРЎЎЈКөСйКТЦРіЈТФ·ПМъРјОӘФӯБПАҙЦЖұёЈ¬ЖдІҪЦиИзПВЈә

Нј1
ІҪЦи1ЈәМъРјөДҙҰАнЎЈҪ«·ПМъРј·ЕИлИИөДМјЛбДЖИЬТәЦРҪюЕЭјё·ЦЦУәуЈ¬УГНј1ЛщКҫ·Ҫ·Ё·ЦАліц№ММеІўПҙөУЎўёЙФпЎЈ
ІҪЦи2ЈәFeSO4ИЬТәөДЦЖұёЎЈҪ«ҙҰАнәГөДМъРј·ЕИлЧ¶РОЖҝЈ¬јУИл№эБҝөД3 molЎӨ
LЈӯ1H2SO4ИЬТәЈ¬јУИИЦБід·Ц·ҙУҰОӘЦ№ЎЈіГИИ№эВЛ(ИзНј2ЛщКҫ)Ј¬КХјҜВЛТәәНПҙөУТәЎЈ

Нј2
ІҪЦи3ЈәБтЛбСЗМъп§өДЦЖұёЎЈПтЛщөГFeSO4ИЬТәЦРјУИлұҘәН(NH4)2SO4ИЬТәЈ¬ҫӯ№эјУИИЕЁЛхЎўАдИҙҪбҫ§Ўў№эВЛЎўТТҙјПҙөУәуөГөҪБтЛбСЗМъп§ҫ§МеЎЈ
Зл»ШҙрПВБРОКМвЈә
(1)ІҪЦи1ЦРНј1·ЦАл·Ҫ·ЁіЖОӘ________·ЁЎЈ
(2)ІҪЦи2ЦРУРТ»ҙҰГчПФІ»әПАнөДКЗ___________________________________ЎЈ
іГИИ№эВЛөДАнУЙКЗ________________________________________________ЎЈ
(3)ІҪЦи3јУИИЕЁЛх№эіМЦРЈ¬өұ________КұНЈЦ№јУИИЎЈУГОЮЛ®ТТҙјПҙөУҫ§МеөДФӯТтКЗ______________________________________________________________ЎЈ
(4)FeSO4ЎӨ7H2OФЪіұКӘөДҝХЖшЦРТЧұ»Сх»ҜіЙFe(OH)SO4ЎӨ3H2OЈ¬РҙіцёГ·ҙУҰөД»ҜС§·ҪіМКҪ________________________________________________________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com