

 O=C=O��2�֣��Ƶ�������VIIA�壬�ȼҵ��2�֣�
O=C=O��2�֣��Ƶ�������VIIA�壬�ȼҵ��2�֣� CaCl2��2NH3����2H2O��2�֣�
CaCl2��2NH3����2H2O��2�֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������Խǿ��������������Ӧˮ����ļ���Խǿ |
| B��������Խǿ�������ʵ����������������ᷴӦ���ɵ�����Խ�� |
| C��������Խǿ���䵥�ʾ�����۵�Խ�� |
| D��������Խǿ�����Ӧ�������ӵ�������Խǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
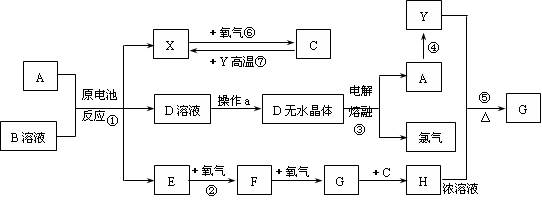
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
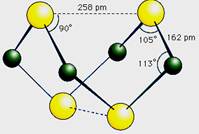
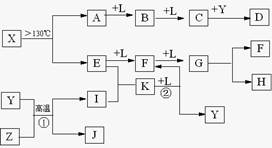
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������һ���������� |
| B��������һ�����ڻ�ѧ�� |
| C�����ʯ��״�ṹ�У��ɹ��ۼ����ɵ�̼ԭ�ӻ��У���С�Ļ�����4��̼ԭ�� |
D������Ԫ��X��Y���γ�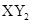 �ͻ������X��Y ��ԭ������֮�����Ϊ2��5 �ͻ������X��Y ��ԭ������֮�����Ϊ2��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��K >Na >Li | B��Na+>Mg2+>Al3+ |
| C��Mg2+>Na+>F�� | D��Cl��>F��>F |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
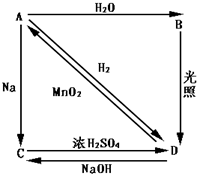
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com