
ĻĀĮŠČÜŅŗÖŠĮ£×ÓµÄĪļÖŹµÄĮæÅØ¶Č¹ŲĻµÕżČ·µÄŹĒ (””””)
A£®0.1 mol”¤L£1 NaHCO3ČÜŅŗÓė0.1 mol”¤L£1 NaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠ£ŗ c(Na£« )£¾c(CO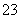 )£¾c(HCO
)£¾c(HCO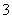 )£¾c(OH£ )
)£¾c(OH£ )
B£®20 mL 0.1 mol”¤L£1 CH3COONaČÜŅŗÓė10 mL 0.1 mol”¤L£1 HClČÜŅŗ»ģŗĻŗóČÜŅŗ³ŹĖįŠŌ£¬ĖłµĆČÜŅŗÖŠ£ŗc(CH3COO£ )£¾c(Cl£ )£¾c(CH3COOH)£¾c(H£«)
C£®ŹŅĪĀĻĀ£¬pH£½2µÄŃĪĖįÓėpH£½12µÄ°±Ė®µČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠ£ŗ c(Cl£)£«c(H£«)£¾c(NH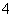 )£«c(OH£)
)£«c(OH£)
D£®0.1 mol”¤L£1 CH3COOHČÜŅŗÓė0.1 mol”¤L£1 NaOHČÜŅŗµČĢå»ż»ģŗĻ£¬ĖłµĆČÜŅŗÖŠ£ŗ c(OH£)£¾c(H£«)£«c(CH3COOH)
½āĪö””AĻī£¬µČĪļÖŹµÄĮæÅØ¶ČµÄNaHCO3ČÜŅŗÓėNaOHČÜŅŗ»ģŗĻµĆµ½Na2CO3ČÜŅŗ£¬ÓÉÓŚCO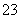 £«H2OHCO
£«H2OHCO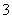 £«OH££¬HCO
£«OH££¬HCO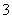 £«H2OH2CO3£«OH££¬H2OH£«£«OH££¬ŌņÓŠc(Na£«)>c(CO
£«H2OH2CO3£«OH££¬H2OH£«£«OH££¬ŌņÓŠc(Na£«)>c(CO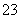 )>c(OH£)>c(HCO
)>c(OH£)>c(HCO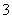 )£¬“ķĪó£»BĻī£¬CH3COONaÓėHCl°“ĪļÖŹµÄĮæ±Č2”Ć1·“Ó¦ŗó£¬ČÜŅŗĪŖµČĪļÖŹµÄĮæÅØ¶ČµÄCH3COONa”¢CH3COOHŗĶNaClµÄ»ģŗĻŅŗ£¬ĻŌĖįŠŌ£¬ĖµĆ÷CH3COOHµÄµēĄė³Ģ¶Č“óÓŚCH3COO£µÄĖ®½ā³Ģ¶Č£¬Ņņ“Ėc(CH3COO£)>c(Cl£)>c(CH3COOH)>c(H£«)£¬ÕżČ·£»CĻī£¬ÓÉÓŚHClŹĒĒæµē½āÖŹ£¬NH3”¤H2OŹĒČõµē½āÖŹ£¬ŌņpH£½2ŃĪĖįµÄÅØ¶ČŠ”ÓŚpH£½12°±Ė®µÄÅØ¶Č£¬µČĢå»ż»ģŗĻŗ󣬰±Ė®¹żĮ棬·“Ó¦ŗóĪŖNH4ClŗĶNH3”¤H2OµÄ»ģŗĻŅŗ£¬ČÜŅŗĻŌ¼īŠŌc(OH£)>c(H£«)£¬øł¾ŻµēŗÉŹŲŗćc(Cl£)£«c(OH£)£½c(NH
)£¬“ķĪó£»BĻī£¬CH3COONaÓėHCl°“ĪļÖŹµÄĮæ±Č2”Ć1·“Ó¦ŗó£¬ČÜŅŗĪŖµČĪļÖŹµÄĮæÅØ¶ČµÄCH3COONa”¢CH3COOHŗĶNaClµÄ»ģŗĻŅŗ£¬ĻŌĖįŠŌ£¬ĖµĆ÷CH3COOHµÄµēĄė³Ģ¶Č“óÓŚCH3COO£µÄĖ®½ā³Ģ¶Č£¬Ņņ“Ėc(CH3COO£)>c(Cl£)>c(CH3COOH)>c(H£«)£¬ÕżČ·£»CĻī£¬ÓÉÓŚHClŹĒĒæµē½āÖŹ£¬NH3”¤H2OŹĒČõµē½āÖŹ£¬ŌņpH£½2ŃĪĖįµÄÅØ¶ČŠ”ÓŚpH£½12°±Ė®µÄÅØ¶Č£¬µČĢå»ż»ģŗĻŗ󣬰±Ė®¹żĮ棬·“Ó¦ŗóĪŖNH4ClŗĶNH3”¤H2OµÄ»ģŗĻŅŗ£¬ČÜŅŗĻŌ¼īŠŌc(OH£)>c(H£«)£¬øł¾ŻµēŗÉŹŲŗćc(Cl£)£«c(OH£)£½c(NH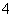 )£«c(H£«)£¬¹Źc(Cl£)£«c(H£«)<c(NH
)£«c(H£«)£¬¹Źc(Cl£)£«c(H£«)<c(NH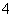 )£«c(OH£)£¬“ķĪó£»DĻī£¬µČĪļÖŹµÄĮæµÄCH3COOHÓėNaOH»ģŗĻ£¬ĖłµĆČÜŅŗµÄČÜÖŹĪŖCH3COONa£¬øł¾ŻÖŹ×ÓŹŲŗćÓŠc(OH£)£½c(H£«)£«c(CH3COOH)£¬“ķĪó”£
)£«c(OH£)£¬“ķĪó£»DĻī£¬µČĪļÖŹµÄĮæµÄCH3COOHÓėNaOH»ģŗĻ£¬ĖłµĆČÜŅŗµÄČÜÖŹĪŖCH3COONa£¬øł¾ŻÖŹ×ÓŹŲŗćÓŠc(OH£)£½c(H£«)£«c(CH3COOH)£¬“ķĪó”£
“š°ø””B


 »ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
»ĘøŌ¹Ś¾üæĪæĪĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
H2SĖ®ČÜŅŗÖŠ“ęŌŚµēĄėĘ½ŗāH2SH£«£«HS£ŗĶHS£H£«£«S2£”£ČōĻņH2SČÜŅŗÖŠ (””””)
A£®¼ÓĖ®£¬Ę½ŗāĻņÓŅŅĘ¶Æ£¬ČÜŅŗÖŠĒāĄė×ÓÅضČŌö“ó
B£®ĶØČė¹żĮæSO2ĘųĢå£¬Ę½ŗāĻņ×óŅĘ¶Æ£¬ČÜŅŗpHÖµŌö“ó
C£®µĪ¼ÓŠĀÖĘĀČĖ®£¬Ę½ŗāĻņ×óŅĘ¶Æ£¬ČÜŅŗpHÖµ¼õŠ”
D£®¼ÓČėÉŁĮæĮņĖįĶ¹ĢĢå(ŗöĀŌĢå»ż±ä»Æ)£¬ČÜŅŗÖŠĖłÓŠĄė×ÓÅØ¶Č¶¼¼õŠ”
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧÓėÉś»īĆÜĒŠĻą¹Ų”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ(””””)
A£®ŅŅĻ©æÉ×÷Ė®¹ūµÄ“ߏģ¼Į B£®¹č½ŗæÉ×÷“ü×°Ź³Ę·µÄøÉŌļ¼Į
C£®ø£¶ūĀķĮÖæÉ×÷Ź³Ę·µÄ±£ĻŹ¼Į D£®ĒāŃõ»ÆĀĮæÉ×÷ĪøĖįµÄÖŠŗĶ¼Į
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚĢå»ż¾łĪŖ1 L£¬pH¾łµČÓŚ2µÄŃĪĖįŗĶ“×ĖįČÜŅŗÖŠ£¬·Ö±šĶ¶Čė0.12 gĆ¾·Ū³ä·Ö·“Ó¦ŗó£¬ĻĀĶ¼ÖŠ±Č½Ļ·ūŗĻ·“Ó¦ŹĀŹµµÄĒśĻߏĒ(””””)
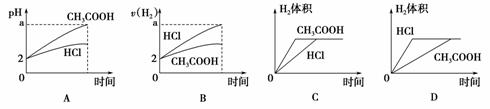
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
³£ĪĀĻĀa mol”¤L£1Ļ”°±Ė®ŗĶb mol”¤L£1Ļ”ŃĪĖįµČĢå»ż»ģŗĻ£¬¶Ō»ģŗĻŗóČÜŅŗÅŠ¶ĻŅ»¶ØÕżČ·µÄŹĒ(””””)
A£®Čōa£½b£¬Ōņc(NH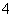 )£½c(Cl£) B£®Čōa£¾b£¬Ōņc(NH
)£½c(Cl£) B£®Čōa£¾b£¬Ōņc(NH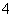 )£¾c(Cl£)
)£¾c(Cl£)
C£®Čōa£¾b£¬Ōņc(OH£)£¾c(H£«) D£®Čōa£¼b£¬Ōņc(OH£)£¼c(H£«)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ±ä»ÆÖŠŹōÓŚ»Æѧ±ä»ÆµÄŹĒ________”£
¢ŁĆŗµÄøÉĮ󔔢ŚÕōĮ󔔢ŪĻš½ŗµÄĄĻ»Æ””¢ÜĆŗµÄĘų»Æ
¢ŻŃęÉ«·“Ó¦””¢Ž¶Ū»Æ””¢ßµē¶Ę””¢ą½ŗĢå¾Ū³Į””¢įŃõĘų×Ŗ»ÆĪŖ³ōŃõ””¢ā137I×Ŗ±äĪŖ131I
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¹ŲÓŚĪļÖŹ·ÖĄąµÄĖµ·ØÕżČ·µÄŹĒ(””””)
A£®½šøÕŹÆ”¢°×Į׶¼ŹōÓŚµ„ÖŹ
B£®ĘÆ°×·Ū”¢ŹÆÓ¢¶¼ŹōÓŚ“æ¾»Īļ
C£®ĀČ»Æļ§”¢“ĪĀČĖį¶¼ŹōÓŚĒæµē½āÖŹ
D£®ĘĻĢŃĢĒ”¢µ°°×ÖŹ¶¼ŹōÓŚøß·Ö×Ó»ÆŗĻĪļ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧÓė׏Ō“”¢»·¾³”¢Éś»ī¹ŲĻµĆÜĒŠ£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A£®æÕĘųÖŠPM2.5(2.5Ī¢Ć×ŅŌĻĀµÄæÅĮ£Īļ)µÄ“ęŌŚÄܹ»ŠĪ³É¶”“ļ¶ūŠ§Ó¦
B£®ĆŗČ¼ÉÕŹ±¼ÓČėÉŁĮæµÄÉśŹÆ»ŅæÉŅŌ¼õÉŁ·ĻĘųÖŠµÄ¶žŃõ»ÆĮņÅÅ·Å
C£®½«ŌģÖ½·ĻĖ®ĶعżøßŃ¹Ė®¾®Ń¹µ½µŲĻĀ£¬½ŚŌ¼Éś²ś³É±¾
D£®ĀĢÉ«»ÆѧµÄŗĖŠÄŹĒÓ¦ÓĆ»ÆѧŌĄķ¶Ō»·¾³ĪŪČ¾½ųŠŠÖĪĄķ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĶʶĻÕżČ·µÄŹĒ ( )
A.SiO2ŹĒĖįŠŌŃõ»ÆĪļ£¬ÄÜÓėNaOHČÜŅŗ·“Ó¦
B£®Na2O”¢Na2O2×é³ÉŌŖĖŲĻąĶ¬£¬ÓėCO2·“Ó¦²śĪļŅ²ĻąĶ¬
C. CO”¢NO”¢NO2¶¼ŹĒ“óĘųĪŪČ¾ĘųĢ壬ŌŚæÕĘųÖŠ¶¼ÄÜĪČ¶Ø“ęŌŚ
D£®ŠĀÖĘĀČĖ®ĻŌĖįŠŌ£¬ĻņĘäÖŠµĪ¼ÓÉŁĮæ×ĻÉ«ŹÆČļŹŌŅŗ£¬³ä·ÖÕńµ“ŗóČÜŅŗ³ŹŗģÉ«
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com