
 £¬ĖüŹĒ¼«ŠŌ£ØĢī”°¼«ŠŌ”±»ņ”°·Ē¼«ŠŌ”±£©¼üŠĪ³ÉµÄ·Ē¼«ŠŌ·Ö×Ó£®
£¬ĖüŹĒ¼«ŠŌ£ØĢī”°¼«ŠŌ”±»ņ”°·Ē¼«ŠŌ”±£©¼üŠĪ³ÉµÄ·Ē¼«ŠŌ·Ö×Ó£®·ÖĪö »ÆŗĻĪļDCĪŖĄė×Ó»ÆŗĻĪļ£¬DµÄ¶ž¼ŪŃōĄė×ÓÓėCµÄŅõĄė×Ó¾ßÓŠĻąĶ¬µÄµē×Ó²ć½į¹¹£¬ĖµĆ÷DŌŚCµÄĻĀŅ»ÖÜĘŚ£¬DÓ¦ĪŖMgŌŖĖŲ£»AC2ĪŖ·Ē¼«ŠŌ·Ö×Ó£¬ŹĒ²śÉśĪĀŹŅŠ§Ó¦µÄÖ÷ŅŖĘųĢ壬øĆĘųĢåÓ¦ĪŖCO2£¬ŌņAĪŖCŌŖĖŲ£¬CĪŖOŌŖĖŲ£¬ŌņBĪŖNŌŖĖŲ£»ČĖĢåȱEŌŖĖŲ»įµĆČķ¹Ē²”£¬DÓėEĪ»ÓŚĶ¬Ö÷×壬ŌņEĪŖCaŌŖĖŲ£¬½įŗĻ¶ŌÓ¦ŌŖĖŲµÄµ„ÖŹŗĶ»ÆŗĻĪļµÄŠŌÖŹŅŌ¼°ĢāÄæŅŖĒóæɽā“šøĆĢā£®
½ā“š ½ā£ŗ»ÆŗĻĪļDCĪŖĄė×Ó»ÆŗĻĪļ£¬DµÄ¶ž¼ŪŃōĄė×ÓÓėCµÄŅõĄė×Ó¾ßÓŠĻąĶ¬µÄµē×Ó²ć½į¹¹£¬ĖµĆ÷DŌŚCµÄĻĀŅ»ÖÜĘŚ£¬DÓ¦ĪŖMgŌŖĖŲ£»AC2ĪŖ·Ē¼«ŠŌ·Ö×Ó£¬ŹĒ²śÉśĪĀŹŅŠ§Ó¦µÄÖ÷ŅŖĘųĢ壬øĆĘųĢåÓ¦ĪŖCO2£¬ŌņAĪŖCŌŖĖŲ£¬CĪŖOŌŖĖŲ£¬ŌņBĪŖNŌŖĖŲ£»ČĖĢåȱEŌŖĖŲ»įµĆČķ¹Ē²”£¬DÓėEĪ»ÓŚĶ¬Ö÷×壬ŌņEĪŖCaŌŖĖŲ£¬
£Ø1£©Ķ¬ÖÜĘŚĖęŌ×ÓŠņŹżŌö“󣬵ŚŅ»µēĄėÄܳŹŌö“óĒ÷ŹĘ£¬NŌŖĖŲ2pÄܼ¶ČŻÄÉ3øöµē×Ó£¬ĪŖ°ėĀśĪȶØדĢ¬£¬ÄÜĮæ½ĻµĶ£¬µŚŅ»µēĄėÄÜøßÓŚĶ¬ÖÜĘŚĻąĮŚŅāĖ¼ŹĒ£¬¹ŹµŚŅ»µēĄėÄÜÓÉŠ”µ½“óµÄĖ³ŠņĪŖN£¾O£¼C£¬
¹Ź“š°øĪŖ£ŗN£¾O£¼C£»
£Ø2£©BĪŖNŌŖĖŲ£¬¶ŌÓ¦µÄĒā»ÆĪļĪŖNH3£¬°±ĘųČÜÓŚĖ®Éś³ÉČõµē½āÖŹŅ»Ė®ŗĻ°±£¬Ņ»Ė®ŗĻ°±µÄµēĄė·½³ĢŹ½ĪŖ£ŗNH3+H2O?NH3•H2O?NH4++OH-£¬
¹Ź“š°øĪŖ£ŗNH3£»NH3+H2O?NH3•H2O?NH4++OH-£»
£Ø3£©AC2ĪŖCO2£¬ĪŖ¹²¼Ū»ÆŗĻĪļ£¬µē×ÓŹ½ĪŖ £¬¶žŃõ»ÆĢ¼ĪŖÖ±ĻߊĶ½į¹¹£¬ŹĒÓɼ«ŠŌ¼üŠĪ³ÉµÄ·Ē¼«ŠŌ·Ö×Ó£¬
£¬¶žŃõ»ÆĢ¼ĪŖÖ±ĻߊĶ½į¹¹£¬ŹĒÓɼ«ŠŌ¼üŠĪ³ÉµÄ·Ē¼«ŠŌ·Ö×Ó£¬
¹Ź“š°øĪŖ£ŗ £»¼«ŠŌ£»
£»¼«ŠŌ£»
£Ø4£©NµÄ×īµĶ¼ŪĪŖ-3£¬DĪŖMgŌŖĖŲ£¬ŌņӦɜ³ÉNH4NO3£¬·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗ4Mg+10HNO3=4Mg£ØNO3£©2+NH4NO3 +3H2O£¬
¹Ź“š°øĪŖ£ŗ4Mg+10HNO3=4Mg£ØNO3£©2+NH4NO3 +3H2O£®
µćĘĄ ±¾Ģāæ¼²é½į¹¹ŠŌÖŹĪ»ÖĆ¹ŲĻµÓ¦ÓĆ£¬ĢāÄæÄѶČÖŠµČ£¬Éę¼°µēĄėÄÜ”¢·Ö×Ó½į¹¹”¢ŌӻƹģµĄ”¢µē×ÓŹ½”¢ÅäŗĻĪļ”¢Ńõ»Æ»¹Ō·“Ó¦µČ£¬Ēā»ÆĪļµÄ·Šµć±ČĖüĆĒĶ¬×åĻąĮŚÖÜĘŚŌŖĖŲĒā»ÆĪļµÄ·ŠµćøߏĒĶʶĻµÄĶ»ĘĘæŚ£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ”÷H1-”÷H2 | B£® | ”÷H1-$\frac{1}{2}$”÷H2 | C£® | 2”÷H1-”÷H2 | D£® | $\frac{1}{2}$”÷H2-”÷H1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
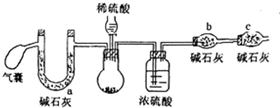 ¹żŃõ»ÆÄĘæÉ×öŗ½Ģģ·É“¬µÄ¹©Ńõ¼Į£¬¶ŌĘä“æ¶ČŅŖĒóŗÜøߣ¬Ä³Š”×éĶ¬Ń§ĪŖĮĖ²ā¶Ø¹żŃõ»ÆÄʵēæ¶Č£ØŌÓÖŹĪŖĢ¼ĖįÄĘ£©£¬Éč¼ĘĮĖČēĻĀ·½°ø£ŗ
¹żŃõ»ÆÄĘæÉ×öŗ½Ģģ·É“¬µÄ¹©Ńõ¼Į£¬¶ŌĘä“æ¶ČŅŖĒóŗÜøߣ¬Ä³Š”×éĶ¬Ń§ĪŖĮĖ²ā¶Ø¹żŃõ»ÆÄʵēæ¶Č£ØŌÓÖŹĪŖĢ¼ĖįÄĘ£©£¬Éč¼ĘĮĖČēĻĀ·½°ø£ŗ| ĒāŃõ»ÆÄĘÅØ¶Č£Ømol•L-1£© | 5 | 2 | 1 | 0.01 |
| ±äŗģŗóĶŹÉ«µÄŹ±¼ä£Øs£© | 8 | 94 | 450 | ³¤Ź±¼ä²»ĶŹÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £®
£®
 £®
£® £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | C3H8 | B£® | C4H10 | C£® | C5H12 | D£® | C6H14 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | C2H6”¢C2H4O | B£® | C2H4O”¢C2H4O2 | C£® | C2H6O”¢C3H6O3 | D£® | C3H8O3”¢C2H4O2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŖĖŲ | B£® | Ō×Ó | C£® | ·Ö×Ó | D£® | µ„ÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ”°°¾µØ·ÆĢśøŖ£¬¾ĆÖ®±ä»ÆĪŖĶ”±£¬øĆ¹ż³Ģ·¢ÉśĮĖÖĆ»»·“Ó¦ | |
| B£® | ”°ö²³¾»ż¾ŪÄŃ¼ūĀ·ČĖ”±£¬Īķö²ĖłŠĪ³ÉµÄĘųČܽŗÓŠ¶”“ļ¶ūŠ§Ó¦ | |
| C£® | ”°ĒąŻļŅ»ĪÕ£¬ŅŌĖ®¶žÉż×Õ£¬½ŹČ”Ö”±£¬ĶĄßĻßĻ¶ŌĒąŻļĖŲµÄĢįČ”ŹōÓŚ»Æѧ±ä»Æ | |
| D£® | ¹Å½£”°ÉņĀ¬”±”°ŅŌ¼ĮøÖĪŖČŠ£¬ČįĢśĪŖ¾„øÉ£¬²»¶ūŌņ¶ą¶ĻÕŪ”±£¬¼ĮøÖÖøµÄŹĒĢśµÄŗĻ½š |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com