
��ѧ�ͻ���������ʳƷӪ��������Ӧ�õ�������ء�
��1�����λ�����Ⱦ��Ӫ�찲ȫ����̬�����ѳ�Ϊȫ����Ĺ�ʶ��
�ٿ�����Ⱦָ���Ǹ��ݿ����� (ѡ�������̼�� ��������������)�����������Ϳ������������Ⱦ���Ũ�ȼ����������ֵ��
����Ȼˮ�����ʽ϶࣬������������������������д�������������ˮ�����ɵ� ��������ˮ�е�����������
�����д�ʩ�����ڸ��ƻ����������� ��(����ĸ)
A����ȼú������ʯ��ʯ��ĩ B�����кͷ���ȥ��ҵ��ˮ�е��� C������Ķѷ���������
��2����������������ͷ�չ�����ʻ���������ʹ�ò��Ͽ��Ը������ǵ������ԭ�ӷ�Ӧ���е��Թ㷺Ӧ�õ��ƼغϽ��ڳ����³�Һ̬��˵���Ͻ���۵������ɷֽ������۵� ��ѡ��ߡ��͡�����
��3�����������彡���������й�ϵ
��1������ʳ�︻��άC���� ������ţ���
��2������������������Ӫ�����ʣ��������������շֽ�Ϊ ������ţ���
A .������ B.֬���� C . ������
 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ÿ���е��������壬�������ü�ʯ�Ҹ�����ǣ� ��
A. H2S��SO2 B. NO2��HBr C. CO2��CO D. NH3��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ף�P4����һ�ֳ����ľ��壬�������Ʊ��ϴ������ᡣ
��1��������_______���壬31g������������������ȫȼ���ͷų�745.5kJ����������д������ȼ�յ��Ȼ�ѧ��Ӧ����ʽ��_________________________________________��
��2����֪����������Һ�ɷ������·�Ӧ��
P4 + HClO3 + === HCl + H3PO4
��ƽ�����������Ӧ����ʽ���÷�Ӧ����������______________��
��3�������ж�����ʵ���ҿɲ���CuSO4��Һ���д������䷴ӦΪ��
12P4 + 60CuSO4 + 96H2O === 20Cu3P + 24 H3PO4 + 60 H2SO4
�÷�Ӧ������������______________������1.1mol P4��Ӧ������________mol����ת�ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������������ͬλ�ص���
A��16O��18O���� B��H2O��D2O C��O2��O3 D��24Mg��24Na
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й����ӵļ��鷽��һ����ȷ����
A����ij��Һ�еμ�BaCl2��Һ�����а�ɫ�������ٵμ�����ϡHNO3�����������ܽ⣬��˵��ԭ��Һ��һ����SO42-
B���ò�˿պȡij��Һ����ɫ����������ֱ�ӹ۲������ɫ��δ����ɫ��˵��ԭ��Һ�в���K+
C����ij��Һ�м�����ϡHCl��������������ʹ����ʯ��ˮ�������˵����Һ��һ����CO32��
D��ij��Һ�м�NaOH��Һ�����Ȳ�����������ʹʪ���ɫʯ����ֽ����˵����Һ��һ����NH4+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪25 �桢101 kPa�£�ʯī�����ʯȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ
C(ʯī��s)��O2(g)===CO2(g) ��H����393.51 kJ��mol��1��
C(���ʯ��s)��O2(g)===CO2(g) ��H����395.41 kJ��mol��1��
�ݴ��жϣ�����˵����ȷ���� (����)
A����ʯī�Ʊ����ʯ�����ȷ�Ӧ��ʯī�������Ƚ��ʯ�ĵ�
B����ʯī�Ʊ����ʯ�����ȷ�Ӧ��ʯī�������Ƚ��ʯ�ĸ�
C����ʯī�Ʊ����ʯ�Ƿ��ȷ�Ӧ��ʯī�������Ƚ��ʯ�ĵ�
D����ʯī�Ʊ����ʯ�Ƿ��ȷ�Ӧ��ʯī�������Ƚ��ʯ�ĸ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ܱ������У�A��B��Ӧ����C���䷴Ӧ���ʷֱ���v(A)��v(B)��v(C)��ʾ����֪3v(B)��2v(A)��2v(C)��3v(B)����˷�Ӧ�ɱ�ʾΪ (����)
A��2A��3B===2C B��A��3B===2C
C��3A��2B===3C D��A��B===C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����ӦFe(s)��CO2(g)  FeO(s)��CO(g)��ƽ�ⳣ��ΪK1��
FeO(s)��CO(g)��ƽ�ⳣ��ΪK1��
��ӦFe(s)��H2O(g)  FeO(s)��H2(g)��ƽ�ⳣ��ΪK2����ͬ�¶�ʱK1��K2��ֵ���±���
FeO(s)��H2(g)��ƽ�ⳣ��ΪK2����ͬ�¶�ʱK1��K2��ֵ���±���
| �¶�/K | K1 | K2 |
| 973 | 1.47 | 2.38 |
| 1 173 | 2.15 | 1.67 |
���ݱ������ݣ����㷴ӦCO2(g)��H2(g)  CO(g)��H2O(g)��973Kʱ��ƽ�ⳣ����K
CO(g)��H2O(g)��973Kʱ��ƽ�ⳣ����K
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�����A��B��C�ֱ�Ϊ���塢����ɫ���塢��ɫ���壬�ں��ʵ������£����ǿ�����ͼ��ʾ��ͼ���з�Ӧ����֪E��Һ����ɫ�ģ���ش�
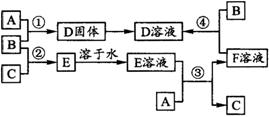
��1��A��________��B��________��C��________�������ѧʽ��
��2����Ӧ�ٵĻ�ѧ����ʽΪ________________��
��3����Ӧ�۵Ļ�ѧ����ʽΪ________________��
��4����Ӧ�ܵĻ�ѧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com