
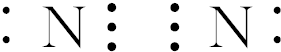 ��
�� ��
�� ��
�� ���� һ����˵�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ�����ͬ�ǽ���Ԫ��֮�����γɼ��Լ���ͬ�ַǽ���Ԫ��֮�����γɷǼ��Լ����������Ӽ��Ļ����������ӻ����ֻ�����ۼ��Ļ������ǹ��ۻ�����ݴ˷������
��� �⣺��N2 ��ֻ���Ǽ��Լ������ڵ��ʣ�
��Na2O2�к������Ӽ��ͷǼ��Լ����������Ӽ������
��NaOH����NH4Cl�к������Ӽ��ͼ��Լ����������Ӽ������
��HCl��ֻ�����Լ������ڹ��ۻ����
��H2O2 ����H-O���Լ���O-O�Ǽ��Լ������ڹ��ۻ����
��Na2S��ֻ�����Ӽ����������ӻ����
�ʴ�Ϊ����1���ޣ�2���ܣ�3���٣�4���ߣ�5���ݣ�6���ۣ�7���ڣ�
��8�����ݵ���ʽ����д�������٢ۢĵ���ʽ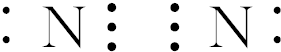 ��
�� ��
�� ��
��
�ʴ�Ϊ��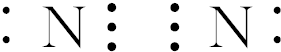 ��
�� ��
�� ��
��
���� ���⿼�������ʺͻ�ѧ���Ĺ�ϵ������ʽ����д����ȷ�����д��ڵĻ�ѧ���ǽⱾ��ؼ�����Ŀ�ѶȲ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ֹ���Ǧ���ӣ��ֹܿɱ����� | |
| B�� | ������Ũ�������ۻ����ɱ����ڲ�������ʴ | |
| C�� | ���������������ױ���ʴ | |
| D�� | �����������ⸯʴʱ��������Ӧ��Fe-3e-�TFe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ܢޢ� | B�� | �ڢݢޢ� | C�� | �٢ۢܢ� | D�� | ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
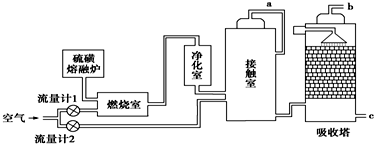
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
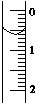 ��ʹ������к͵ζ����ⶨ���۰״���������g•100mL-1����
��ʹ������к͵ζ����ⶨ���۰״���������g•100mL-1����| �ζ�����ʵ�����ݣ�mL�� | 1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.95 | 15.00 | 15.05 | 14.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
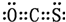 ��̼ԭ�Ӻ�����ӵĹ������ʽΪ
��̼ԭ�Ӻ�����ӵĹ������ʽΪ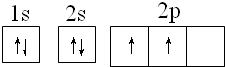 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
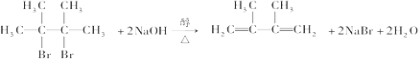 ��
��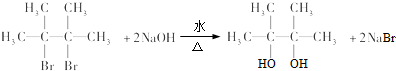 ��E���Ҷ����Ĺ�ϵ��ͬϵ�
��E���Ҷ����Ĺ�ϵ��ͬϵ� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Y��N������������У�Y��N����ԭ��֮���Ϊ˫�� | |
| B�� | һ�������£�Y�������û���N���ʣ�M���û���X���� | |
| C�� | NԪ��λ��Ԫ�����ڱ��е�3����I V�� | |
| D�� | ����Ԫ�ص�ԭ�Ӱ뾶��X��Y��M��N |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH��OH��CH3 | B�� | CH3CH2CH2CH2OH | C�� | CH3CH2CH2OH | D�� | CH3CH2OCH2CH3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com