
 ������ԭ�ζ�ʵ��������к͵ζ����ƣ�����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮��������0.1000mol•L-1KMnO4������Һ�ζ�δ֪Ũ�ȵ���ɫH2C2O4��Һ����Ӧ���ӷ���ʽ�ǣ�2MnO${\;}_{4}^{-}$+5H2C2O4+6H+�T2Mn2++CO2��+8H2O��
������ԭ�ζ�ʵ��������к͵ζ����ƣ�����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮��������0.1000mol•L-1KMnO4������Һ�ζ�δ֪Ũ�ȵ���ɫH2C2O4��Һ����Ӧ���ӷ���ʽ�ǣ�2MnO${\;}_{4}^{-}$+5H2C2O4+6H+�T2Mn2++CO2��+8H2O��| �ζ����� | ����H2C2O4��Һ�����/mL | 0.1000 mol•L-1 KMnO4�����/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 2 8.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
���� ��1�����Ը�����ؾ���ǿ�����ԣ�ʵ��ʱӦ����ʽ�ζ��ܣ���ɫH2C2O4��Һ�����ԣ�����ʽ�ζ��ܣ��ζ����̻���Ҫ�ձ�����ƿ����ֽ���ζ��ܼк�����̨��
��2��������ؾ���ǿ�����ԣ��ܸ�ʴ��ʽ�ζ����¶��ܣ�
��3�����������Һ��������ɫ��Ϊ��ɫ���ڿ�ʼ���������ʱ����ԭ����ɫ��ʧ�����ﵽ�ζ��յ�ʱ���������һ�θ��������Һ��ɫ����ȥ����ҺӦ��������ɫ��Ϊdz��ɫ��
��4�����ݵζ��ܵĽṹ�;�ȷ�ȶ����ζ����еĶ�����
��5���ȸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V��KMnO4�����������ù�ϵʽ��2MnO4-��5H2C2O4����H2C2O4�����ʵ���Ũ�ȣ�
��6������c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��� �⣺��1�����Ը�����ؾ���ǿ�����ԣ�ʵ��ʱӦ����ʽ�ζ��ܣ���ɫH2C2O4��Һ�����ԣ�����ʽ�ζ��ܣ��ζ����̻���ҪҪ�ձ�����ƿ����ֽ���ζ��ܼк�����̨��
�ʴ�Ϊ��ADE��
��2��������ؾ���ǿ�������ܸ�ʴ��ʽ�ζ����е��ܣ����Բ����ü�ʽ�ζ���ʢ�Ÿ��������Һ��Ӧ������ʽ�ζ��ܣ�
�ʴ�Ϊ�������KMnO4��Һ�ḯʴ��ʽ�ζ����¶˽��ܣ�
��3�����������Һ��������ɫ��Ϊ��ɫ���ڿ�ʼ���������ʱ����ԭ����ɫ��ʧ�����ﵽ�ζ��յ�ʱ���������һ�θ��������Һ��ɫ����ȥ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ�����������һ�α�Һʱ����Һ����ɫ����Ϻ�ɫ���Ұ�����ڱ��ֲ���ɫ��
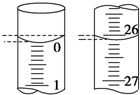
��4����ʽ�ζ����е�Һ����ͼ��ʾ����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��
�ʴ�Ϊ��0.00��26.10��
��5���������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V��KMnO4��=$\frac{26.11+26.09}{2}$mL=26.10mL���ɹ�ϵʽ��2MnO4-��5H2C2O4��n��H2C2O4��=$\frac{5}{2}$n��MnO4-��������c��H2C2O4����0.025L=$\frac{5}{2}$��0.1000mol•L-1��0.02610L�����c��H2C2O4��=0.2610 mol•L-1��
�ʴ�Ϊ��0.2610 mol•L-1��
��6��A����ʽ�ζ���δ�ñ�Һ��ϴ��ֱ��ע��KMnO4��Һ����Һ��Ũ��ƫС�����V������ƫ����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c������ƫ��A����
B���ζ�ǰʢ�Ų�����Һ����ƿ������ˮϴ����û�и����V��������Ӱ�죬����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c���������䣬��B����
C����ʽ�ζ��ܼ��첿���ڵζ�ǰû�����ݣ��ζ��������ݣ����V������ƫС������c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c������ƫС����C��ȷ��
D����ȡKMnO4��Һʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c������ƫС����D��ȷ��
��ѡCD��
���� ������Ҫ����������ԭ�ζ�ʵ�飬��Ŀ�Ѷ��еȣ���ȷ�к͵ζ��IJ�������Ϊ���ؼ���ע�����к͵ζ��������������뼼�ɣ�����������ѧ���ķ�����������ѧ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �û��������ڷ����� | |
| B�� | �û��������ʽΪC20H24O2 | |
| C�� | 1mol˫��A�������4molBr2 | |
| D�� | ˫��A��ʹ��ˮ��ɫ��������ʹ���Ը��������Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �϶���Na+��Al3+��Mg2+��SO42- | B�� | �϶���Na+��Mg2+��Al3+��Cl- | ||
| C�� | �϶�û��Mg2+��HCO3-��MnO4-��Cl- | D�� | �϶�û��K+��NH4+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
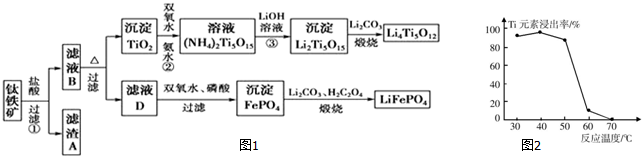
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
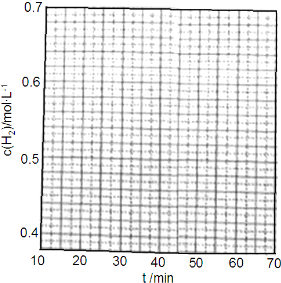
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
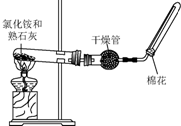 A��B��C��D����ѧ��ѧ�������������ʣ�����֮����ת����ϵ���£����ַ�Ӧ������������ȥ����
A��B��C��D����ѧ��ѧ�������������ʣ�����֮����ת����ϵ���£����ַ�Ӧ������������ȥ�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com