
 Cl2��+H2��+2NaOH��
Cl2��+H2��+2NaOH�� CaSiO3+CO2
CaSiO3+CO2 CaSiO3+CO2
CaSiO3+CO2


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ������ѧ������������ѧ�Ծ��������棩 ���ͣ������
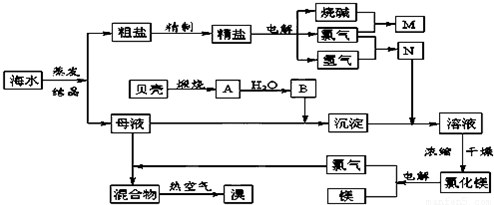
��ˮ�к��м�Ϊ�ḻ����Ȼ��Դ���ܶೣ���Ľ���Ԫ�غͷǽ���Ԫ����Ҫ�Լ����ӵ���ʽ�����ں�ˮ�У���ˮ�������ԡ��ش��������⣺
��ˮ�������һ�������������Ũ���ĺ�ˮ��ͨ��Cl2�������е�Br-������2Br-+Cl2=Br2+2Cl-�������ȿ��������壻��Ȼ���ñ���̼������Һ�����壬���绯ΪNaBr��NaBrO3��������������ữ5NaBr+NaBrO3+3H2SO4=3Na2SO4+3Br2+3H2O
��1��д���ڢڲ���Ӧ�����ӷ���ʽ ��
��2����ˮŨ����Ҫ��һ����������ͨ��Cl2�������ķ����� ��Ŀ���� �����������������������ữ��ԭ������� ��
��3��Ϊ�˳�ȥ��ҵBr2������Cl2,����ҵBr2�� ��
a��ͨ��HBr b������Na2CO3��Һ c������NaBr��Һ d������Na2SO3��Һ
��4����ˮ�����ԭ�������˻�ѧ��ҵ�С����������Ŵ�˼�롣�Ժ�ˮΪԭ���Ʊ�����þ����������д���䷴Ӧԭ�����û�ѧ����ʽ��ʾ��
�� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
±��Ԫ�أ�9F��17Cl��35Br��53I��85At������Ҫ�ķǽ���Ԫ�أ����ǵĵ��ʼ��仯���ﲻ��������ѧ���м���㷺��Ӧ�ã��������л���ѧ��Ҳ���ֲ��ס���ش������й����⣺
��1��ָ����Ԫ����Ԫ�����ڱ��е�λ�� ��
��2�����й���±��Ԫ�ص���������ȷ���� ������ĸ����
A��±�ص��ʵ���ɫ��ԭ�������ĵ�������dz����
B��±����ķе���ԭ�������ĵ������ɵ͵���
C�����Ƶ���ˮ�μӵ��⻯����Һ�У��ٵμӵ�����Һ����ɫ��˵����Ԫ�طǽ����Աȵ�ǿ
D���Ȼ�����Һ�л��������Ȼ��������ɵμ�������ˮ��ȥ
��3����������ѧ���л���Ӧ���û�ѧ����ʽ��ʾ���л����������������±��ԭ�ӵķ���
�� ����һ��±��ԭ�ӣ�������ȡ����Ӧ�� ��
�� ��������±��ԭ�ӣ������ڼӳɷ�Ӧ�� ��
�� �����ĸ�±��ԭ�ӣ������ڼӳɷ�Ӧ�� ��
��4����ˮ�к��зḻ��±�أ�������ȡ���һ�ֹ��շ�������Ԥ�Ⱦ����ữ��Ũ����ˮ��ͨ������ʹ������ת���ɵ����壬�̶�ͨ�������ˮ���������崵����������ʹ�����������ռ�SO2��������ת�����������Դﵽ������Ŀ�ġ�Ȼ�������������������õ���Ʒ�塣
�� д���ڶ���ת�������ӷ���ʽ�� ��
�� �ô˷��Ӻ�ˮ����ȡ1mol�嵥�ʣ��ܹ���Ҫ���� mol��
(5) ijУ̽��С�齫������ĩ״�������������ˮ�У��������Ƿ���ʧ������������ǿ�����ɡ�д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com