
����Ŀ����⾫��ͭ������������Ҫ��Ag��Au�ȹ��ؽ����������ǴӾ���ͭ���������л��������������ͼ��
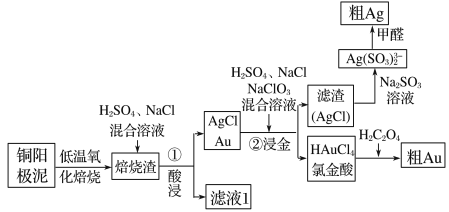
(1)�Ƚ���(HAuCl4)�е�Au�Ļ��ϼ�Ϊ________��
(2)ͭ����������ʱ�����á����±��ա��������á����±��ա���ԭ����_____________________��
(3)�����������ڡ��������ʱ������Ӧ�����ӷ���ʽΪ______________________________________��
(4)���ڽ��𡱷�Ӧ�У�H2SO4������Ϊ___________________________________________���ò���ķ�������У���Ҫ�����õ�AgCl����ˮϴ����������ж�AgCl�Ѿ�ϴ�Ӹɾ��� _____________________________________________________��
(5)�Ƚ���(HAuCl4)��pHΪ2��3�������±����ỹԭΪAu��ͬʱ�ų�������̼���壬��÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________________________________��
(6)��ȩ��ԭ����������ͨ�����ڽ����������¼������������½��У���ȩ������Ϊ̼��������ӣ���÷�Ӧ�����ӷ���ʽΪ___________________________________________��
���𰸡���3 ���±���ʱ�����ɵ�Ag2O�ַֽ�ΪAg��O2(��2Ag2O![]() 4Ag��O2��) Ag2O��2H����2Cl��=2AgCl��H2O �ṩH������ǿNaClO3�������� ȡ���һ��ϴ��Һ�������Թ��У�����Ba(NO3)2��Һ����û�а�ɫ�������������Ѿ�ϴ�Ӹɾ�����֮������Ҫ����ϴ�� 2HAuCl4��3H2C2O4=2Au����8HCl��6CO2�� 4Ag(SO3)23-��HCHO��5OH��=4Ag����8SO32-��3H2O��HCO3-
4Ag��O2��) Ag2O��2H����2Cl��=2AgCl��H2O �ṩH������ǿNaClO3�������� ȡ���һ��ϴ��Һ�������Թ��У�����Ba(NO3)2��Һ����û�а�ɫ�������������Ѿ�ϴ�Ӹɾ�����֮������Ҫ����ϴ�� 2HAuCl4��3H2C2O4=2Au����8HCl��6CO2�� 4Ag(SO3)23-��HCHO��5OH��=4Ag����8SO32-��3H2O��HCO3-
��������
ͭ����������������գ�Ȼ�����������Ȼ��ƵĻ������ˣ�����ΪAgCl��Au���������м����ᡢNaCl�������ƵĻ�����������ΪAgCl����ҺΪ�Ƚ��ᣬAgCl�м�Na2SO3��Һת��ΪAg��SO3��23-������HCHO��ԭ�õ���Ag��HAuCl4��Һ�м�H2C2O4���ɴֽ�
��1���Ƚ��ᣨHAuCl4����ClΪ-1�ۣ�HΪ+1�ۣ���Au�Ļ��ϼ�Ϊ+3���ʴ�Ϊ��+3��
��2�����±���ʱ��Ag������ת��ΪAg2O������ʱ���������ֽ�������Ag���������ʴ�Ϊ�����±���ʱ�����ɵ�Ag2O�ַֽ�ΪAg��O2(��2Ag2O![]() 4Ag��O2��)��
4Ag��O2��)��
��3�����ʱ�������������ᷴӦ�����Ȼ�������Ӧ�����ӷ���ʽΪ��Ag2O+2H++2Cl-=2AgCl+H2O���ʴ�Ϊ��Ag2O+2H++2Cl-=2AgCl+H2O��
��4�����ڽ�������Ӧ�У����������£�Au�������Ʒ�Ӧ�����������ṩH+����ǿNaClO3�������ԣ���Һ�к�����������ӣ��������һ��ϴҺ���Ƿ�����������ӣ������Ϊȡ���һ��ϴ��Һ�������Թ��У�����Ba��NO3��2��Һ����û�а�ɫ�������������Ѿ�ϴ�Ӹɾ�����֮������Ҫ����ϴ�ӣ��ʴ�Ϊ���ṩH������ǿNaClO3�������� ��ȡ���һ��ϴ��Һ�������Թ��У�����Ba(NO3)2��Һ����û�а�ɫ�������������Ѿ�ϴ�Ӹɾ�����֮������Ҫ����ϴ�ӣ�
��5���Ƚ�������ᷴӦ����Au��HCl�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��2HAuCl4+3H2C2O4=2Au+8HCl+6CO2�����ʴ�Ϊ��2HAuCl4+3H2C2O4=2Au+8HCl+6CO2����
��6�������������£�Ag��SO3��23-����ȩ��ԭΪAg��ͬʱ����̼��������ӣ���Ӧ�����ӷ���ʽΪ��4Ag��SO3��23-+HCHO+5OH-=4Ag+8SO32-+3H2O+HCO3-��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ̽��Cu��NO�ķ�Ӧ���������ϣ���Cu��NO��Ӧ������CuO��N2�������������£�NO��NO2�C������MnO4�C��Ӧ����NO3�C��Mn2+
��1��ʵ��������Cu��ϡHNO3�Ʊ�NO��д����Ӧ�Ļ�ѧ����ʽ_____________��
��2��ѡ����ͼ��ʾװ�����Cu��NO��ʵ�顣(�г�װ����) ʵ�鿪ʼǰ����װ����ͨ��һ��ʱ���N2���ش��������⣺

��ʹ��ͭ˿���ŵ���_____________________װ��E������Ϊ_______________��
��װ��C��ʢ�ŵ�ҩƷ������_________��
��װ��D�е�������_______________��װ��F�з�Ӧ�����ӷ���ʽ��_______________��
��3���ⶨNaNO2��NaNO3 �����Һ��NaNO2��Ũ�ȡ� ȡ25.00mL�����Һ����ƿ�У���0.1000mol��L��1����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
����� | 1 | 2 | 3 | 4 |
����KMnO4��Һ���/mL | 20.90 | 20.12 | 20.00 | 19.88 |
����һ��ʵ�����ݳ����쳣����������쳣��ԭ�������_________������ĸ���ţ���
a����ƿϴ����δ����
b����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
c���ζ��յ�ʱ���Ӷ���
d������KMnO4��Һ�к��������������Լ�
e����ƿϴ�����ô���Һ��ϴ
������KMnO4��Һ�ζ�����������Һ�����ӷ���ʽΪ___________________��
��NaNO2 �����ʵ���Ũ��Ϊ__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������![]() ��
��![]() ��FeO��
��FeO��![]() ��
��![]() �м�������������Һ���ֱ�ȡ��ȡҺ�������м���ָ�����ʣ���Ӧ�����Һ����Ҫ���ڵ�һ��������ȷ���ǣ� ��
�м�������������Һ���ֱ�ȡ��ȡҺ�������м���ָ�����ʣ���Ӧ�����Һ����Ҫ���ڵ�һ��������ȷ���ǣ� ��
A.ͨ�����![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]()
B.ͨ�����������![]() ��
��![]() ��
��![]() ��
��![]()
C.�������NaOH��Һ��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]()
D.�������NaClO��Һ��![]() ��
��![]() ��
��![]() ��
��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��Դ���ճ�����߿Ƽ������ж��й㷺Ӧ�á�����˵������ȷ����
A.  Zn2+��Cu�缫�����ƶ���Cu�缫������Һ��H+Ũ������
Zn2+��Cu�缫�����ƶ���Cu�缫������Һ��H+Ũ������
B.  �����ĵ缫��ӦʽΪAg2O��2e��H2O2Ag��2OH
�����ĵ缫��ӦʽΪAg2O��2e��H2O2Ag��2OH
C.  пͲ������������������Ӧ��пͲ��䱡
пͲ������������������Ӧ��пͲ��䱡
D.  ʹ��һ��ʱ��������Һ�����Լ��������������½�
ʹ��һ��ʱ��������Һ�����Լ��������������½�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��������������ԭ�����͵���
A. ����ơ��ƿ��ƿ�����Ϸ��������ĭ
B. H2��I2��HI��������ѹ����ɫ����
C. ����ɫ��NO2��ѹ����ɫ�ȱ����ٱ�dz
D. ��ҵ����������Ĺ�����ʹ�ù����Ŀ���������߶��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ�ת���ڸ��������²���ʵ�ֵ���( )
A.NH3![]() NO
NO![]() HNO3
HNO3
B.Ũ����![]() Cl2
Cl2![]() Ư��
Ư��
C.Al2O3![]() AlCl3(aq)
AlCl3(aq) ![]() ��ˮAlCl3
��ˮAlCl3
D.������![]() C2H5OH
C2H5OH![]() CH3CHO
CH3CHO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��a��b�Ƕ��ʯī�缫��ijͬѧ��ͼʾװ�ý�������ʵ�飺�Ͽ�K2���պ�K1һ��ʱ�䣬�۲쵽��֧�������ڶ������ݽ��缫��Χ����ʱ�Ͽ�K1���պ�K2���۲쵽������A��ָ����ƫת������˵������ȷ����(����)

A.�Ͽ�K2���պ�K1һ��ʱ�䣬��Һ��pH���
B.�Ͽ�K1���պ�K2ʱ��b���ϵĵ缫��ӦΪ2H����2e��=H2��
C.�Ͽ�K2���պ�K1ʱ��a���ϵĵ缫��ӦΪ4OH����4e��=O2����2H2O
D.�Ͽ�K1���պ�K2ʱ��OH����b���ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������£�KMnO4��H2C2O4����������ԭ��Ӧ����0.1 mol��L1��KMnO4�������H2C2O4��Ӧ�����Mn2������������v��ʱ��t��������ͼ������˵����ȷ����(����)

A. �÷�Ӧ��ÿ����1 mol CO2ת�Ƶ���Ϊ10 mol
B. ����ʽ��ƽ��H2O��ϵ��Ϊ6
C. ��Ӧ��ʼ�ܶ�һ��ʱ����v��С����Ϊ��Ӧ��Ũ�ȼ�С������ͻȻ��������Ϊ���ɵ�Mn2���Ը÷�Ӧ�д�����
D. t0ʱ��Mn2����Ũ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ2CH3OH��3O2��4KOH===2K2CO3��6H2O
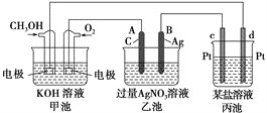
��ش�
(1)�׳���________�أ�ͨ��O2�ĵ缫��Ϊ________�����缫��ӦʽΪ__________��
(2)�ҳ���________�أ�A�缫����Ϊ________�����缫��ӦʽΪ_______________���ҳ��е��ܷ�Ӧ���ӷ���ʽΪ_____________________________________________����Һ��pH________(���������С�����䡱)��
(3)���ҳ���B(Ag)������������5.40 gʱ���׳�������������O2________mL(��״����)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com