
����12�֣���ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d ����ʯī�缫��ͨ��һ��ʱ�����c��d����������������Ϲ��ռ���336mL����״̬�����塣�ش�

��1��ֱ����Դ�У�MΪ ������1�֣�
��2��Pt�缫�����ɵ������� ��������Ϊ __g����1��+2�֣�
��3����Դ����ĵ��ӵ����ʵ�����缫b��c��d�ֱ����ɵ����ʵ����ʵ���֮��Ϊ��2�������� �� ������2�֣�
��4��AgNO3��Һ��Ũ�� �����������С�����䡱����ͬ����AgNO3��Һ��pH ��H2SO4��Һ��Ũ�� ��H2SO4��Һ��pH _����ÿ��1�֣�
��5����H2SO4��Һ�����ʵ�����������5.00%��Ϊ5.02%����ԭ��5.00%��H2SO4��Һ����Ϊ g����2�֣�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����14�֣���ͼ�У�AΪ�����г��������嵥�ʡ�B��C��E�ǽ������ʣ�DΪ�ǽ������ʡ���֪����I��һ�ֳ������������壬Eԭ�Ӻ�����12�����Ӣڷ�Ӧ�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ��ش��������⣺
��1���ֱ�д��F��G��H��I�Ļ�ѧʽ
F G H I
��2����д���л�ѧ����ʽ
��
��3��C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��Ӧ����Һ�����������I��Ӧ�Ļ�ѧ����ʽΪ ��
�� 1.6gG�������ᣬ�õ�����Һ��ͭ����ȫ��Ӧ���������������ͭ�۵�����Ϊ��
����ʾ ��2FeCl3 + Cu ==2FeCl2+ CuCl2 ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����12�֣���ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d ����ʯī�缫��ͨ��һ��ʱ�����c��d����������������Ϲ��ռ���336mL����״̬�����塣�ش�
��1��ֱ����Դ�У�MΪ ������1�֣�
��2��Pt�缫�����ɵ������� ��������Ϊ __g����1��+2�֣�
��3����Դ����ĵ��ӵ����ʵ�����缫b��c��d�ֱ����ɵ����ʵ����ʵ���֮��Ϊ��2�������� �� ������2�֣�
��4��AgNO3��Һ��Ũ�� �����������С�����䡱����ͬ����AgNO3��Һ��pH ��H2SO4��Һ��Ũ�� ��H2SO4��Һ��pH _����ÿ��1�֣�
��5����H2SO4��Һ�����ʵ�����������5.00%��Ϊ5.02%����ԭ��5.00%��H2SO4��Һ����Ϊ g����2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ��У��һ��ѧ����ĩ������ѧ�Ծ� ���ͣ������
����14�֣���ͼ�У�AΪ�����г��������嵥�ʡ�B��C��E�ǽ������ʣ�DΪ�ǽ������ʡ���֪����I��һ�ֳ������������壬Eԭ�Ӻ�����12�����Ӣڷ�Ӧ �ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ��ش��������⣺
�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ��ش��������⣺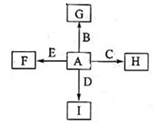
��1���ֱ�д��F��G��H��I�Ļ�ѧʽ
F G H I
��2����д���л�ѧ����ʽ ��
��
��3��C��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��Ӧ����Һ�����������I��Ӧ�Ļ�ѧ����ʽΪ ��
��1.6gG�������ᣬ�õ�����Һ��ͭ����ȫ��Ӧ���������������ͭ�۵�����Ϊ��
����ʾ ��2FeCl3 + Cu ==2FeCl2+ CuCl2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����12�֣���ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d ����ʯī�缫��ͨ��һ��ʱ�����c��d����������������Ϲ��ռ���336mL����״̬�����塣�ش�

��1��ֱ����Դ�У�MΪ ����
��2��Pt�缫�����ɵ������� ��������Ϊ __g����1��+2�֣�
��3����Դ����ĵ��ӵ����ʵ�����缫b��c��d�ֱ����ɵ����ʵ����ʵ���֮��Ϊ��2�������� �� ����
��4��AgNO3��Һ��Ũ�� �����������С�����䡱����ͬ����AgNO3��Һ��pH ��H2SO4��Һ��Ũ�� ��H2SO4��Һ��pH _����ÿ��1�֣�
��5����H2SO4��Һ�����ʵ�����������5.00%��Ϊ5.02%����ԭ��5.00%��H2SO4��Һ����Ϊ g��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com